Book traversal links for A2.1 Information sheet: Practical considerations for implementation of the Abbott RealTime MTB and Abbott RealTime MTB RIF/INH tests
Abbott Molecular diagnostic solution for tuberculosis (TB) has two nucleic acid amplification tests (NAATs), one for detection of Mycobacterium tuberculosis complex (MTBC) (RealTime MTB test) and one for detection of resistance to both rifampicin (RIF) and isoniazid (INH) (RealTime MTB RIF/INH) (1). TB detection is based on the IS6110 genetic element and the pab gene targets. The RIF and INH resistance test uses eight dye-labelled probes to detect mutations in the RIF-resistance determining region (RRDR) of the rpoB gene for RIF resistance and four probes to detect INH resistance, with two probes each for the katG and inhA genes. The test is performed on the m2000 platform, m2000sp instrument used for automated DNA extraction and the m2000rt for performing real-time polymerase chain reaction (PCR). The World Health Organization (WHO) includes these tests within the class of moderate complexity automated NAATs, and the recommendations below apply to these tests (2).
WHO recommendations for use
In people with signs and symptoms of pulmonary TB, moderate complexity automated NAATs may be used on respiratory samples for detection of pulmonary TB, RIF resistance and INH resistance, rather than culture and phenotypic drug susceptibility testing (DST). (Conditional recommendation; moderate certainty of evidence for diagnostic accuracy)
There are several subgroups to be considered for this recommendation:
- The recommendation is based on evidence of diagnostic accuracy in respiratory samples of adults with signs and symptoms of pulmonary TB.
- This recommendation applies to people living with HIV/AIDS (People with HIV) (studies included a varying proportion of such people). Performance on smear-negative samples was reviewed but was only available for TB detection and not for resistance to RIF and INH. Data stratified by HIV status were not available.
- This recommendation applies to adolescents and children based on the generalization of data from adults. Indeterminate results are more likely to be found with paucibacillary TB disease in children.
- Extrapolation for use in people with extrapulmonary TB and testing on non-sputum samples was not considered because data on diagnostic accuracy of technologies in the class for non-sputum samples were limited.
Key performance conclusions
- Both RealTime MTB and RealTime MTB RIF/INH assays perform well for the diagnosis of TB and drug-resistant TB (DR-TB) compared with culture and phenotypic DST.
- Limit of detection reported by the company: TB detection = 17 colony forming units (CFU)/ mL, RIF/INH detection = 60 CFU/mL(3-5).
- The pooled sensitivity and specificity data for the class are presented in the Web Appendix of the WHO consolidated guidelines (6).
Test procedures at-a-glance
The automated RealTime MTB assay targets DNA insertion element IS6110 and the pab genes from eight members of the MTBC (M. tuberculosis, M. africanum, M. bovis, M. bovis BCG, M. microti, M. caprae, M. pinnipedii and M. canetti), using real-time PCR technology to detect the presence of MTBC. For RealTime MTB RIF/INH testing, raw or processed sputum specimens may be inactivated and prepared, or DNA eluates from RealTime MTB-positive specimens may be used directly for testing. The RealTime MTB RIF/INH assay uses real-time PCR-based fluorescent probe technology to detect RIF resistance (rpoB gene), and high-level (katG gene) and low-level (inhA promoter region) INH resistance. Use of both assays together allows for detection of MTBC with or without rifampicin-resistant TB (RR-TB), isoniazid-resistant, rifampicin-susceptible TB (Hr-TB) or multidrug-resistant TB (MDR-TB) within 10.5 hours.
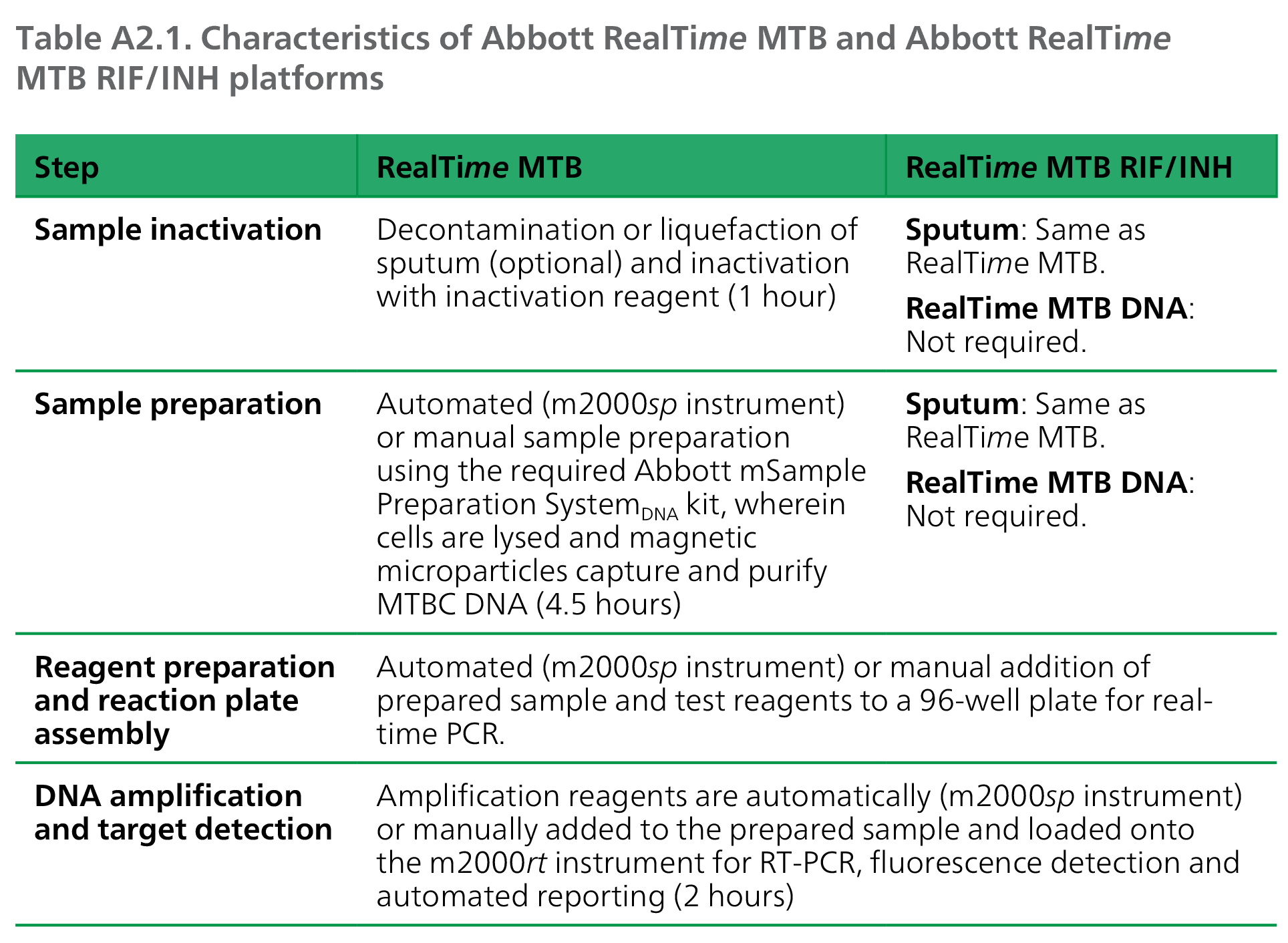
Equipment, supplies and reagents required

Supplied:
RealTime MTB Reagents: RealTime MTB Amplification Reagent Kit (one reagent pack and an internal control) and the RealTime MTB Control Kit (includes positive and negative internal controls).
RealTime MTB RIF/INH Reagents: RealTime MTB RIF/INH Amplification Reagent Kit (includes three reagent packs, an internal control, a positive control and a negative control) and the RealTime MTB RIF/ INH Control Kit (includes external positive and negative controls).
Other reagents: Abbott mSample Preparation SystemDNA Kit (192 sample preparations/kit) and inactivation reagent (IR).
m2000 system: m2000sp instrument (optional for automated, medium throughput sample processing, 145.0 x 79.4 x 217.5 cm, weight 314.4 kg), m24sp instrument (optional for automated, low throughput sample processing, 88.1 x 75.9 x 69.6 cm, weight 84 kg) and m2000rt instrument (required for RT-PCR amplification, fluorescence detection and result reporting, 34 x 49 x 45 cm, weight 34.1 kg).
Not supplied but required
- Class II Biological Safety Cabinet, 2000 x g plate centrifuge and vortex
- Calibrated precision pipettes, aerosol barrier pipette tips, sample racks, reaction vessels, tubes and microcentrifuge tubes
- 95-100% ethanol, 10 M NaOH, isopropanol, Tween20 and molecular water
- Abbott 96-well optical reaction plates and adhesive plate covers with applicator
- Abbott 96-deep-well plates and plate holder(s)
- Personal protective equipment (e.g. chemical-resistant gloves, laboratory coat and eye protection)
Operational considerations
- Testing capacity: RealTime MTB assay (96 samples per kit, including two assay controls), RealTime MTB RIF/INH assay (24 samples per kit, including two assay controls).
- Service and maintenance: Abbott m2000sp (daily maintenance), Abbott m2000rt (weekly and monthly maintenance) and Abbott m2000 system (annual, manufacturer-provided with contract).
- Storage conditions: RealTime MTB amplification and control reagents (-15 °C to -25 °C storage; 2-8 °C after opening), partially used packs must be capped and kept upright, protected from light and used within 14 days. Abbott RealTime MTB RIF/INH Resistance Amplification Reagent Kit (-15 °C to -25 °C storage and after opening), partially used packs must be used within 90 days. Abbott m2000 instrumentation (15-28 °C, relative humidity 30-80%).
- Connectivity: The instruments can be connected, and data exported, to the laboratory information management service. The m2000 system does not have a USB port enabled for connectivity, so data must be exported manually using a CD-ROM.
- Shelf life: Abbott RealTime MTB amplification reagent (90 days from the date of manufacture / 60 days from date of shipment), Abbott RealTime MTB RIF/INH reagents (90 days from date of manufacture / 30 days from date of shipment).
- Unit price: Global prices are not yet available through the Stop TB Partnership Global Drug Facility, although discussions are underway. Abbott is currently offering prices based on order size. Reagent rental agreements may be considered with committed test volumes.
Implementation considerations
In addition to the general guidance provided in Section 3.5 of the main text, consider the following test-specific implementation considerations:
- Area 1 - Policies and planning: Given Abbott m2000 infrastructure requirements and the moderate complexity of the Realtime tests, countries may prioritize instrument placement at a national or central reference laboratory for testing single or multiple diseases (7). Alternatively, countries may have adequate infrastructure available and sufficient sample volume to consider deployment at regional referral laboratories.
The Abbott m2000 system that runs both Realtime TB tests is capable of multi-disease testing, which may be considered for holistic patient testing and potential cost savings across disease programmes (8). Abbott RealTime assays are available for HIV: HIV-1 viral load (VL) and HIV-1 quantitative; for hepatitis C virus (HCV): HCV VL and HCV guideline test (GT); for hepatitis B virus (HBV): HBV VL; for cytomegalovirus (CMV); for Epstein-Barr virus (EBV); for Chlamydia trachomatis and Neisseria gonorrhoeae (CT/NGF); for high-risk human papillomavirus (HPV); and for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although the Abbott m2000sp system requires batching per assay protocol, assays for HIV-1 and HCV, as well as those for CMV and EBV, can be run on the same plate.
- Area 3 - Equipment: Abbott m2000 instruments have high infrastructure requirements and should be placed at laboratories that can accommodate molecular workflow, including separate and dedicated preparation and amplification spaces (one for sample preparation and one for amplification via real-time PCR). If automated sample preparation is desired, the m2000sp instrument must be procured and maintained through regular service in the sample preparation area.
- Area 5 - Procedures: If desired, RealTime MTB assay DNA eluant may be used for reflex testing with the RealTime MTB RIF/INH assay on the m2000 system, to save sample processing and inactivation time. The RealTime MTB RIF/INH assay uses mutations in the rpoB gene, katG gene and inhA promoter region of MTBC DNA to detect resistance to RIF and INH. Based on level of detection of wild-type probes (rpoB, katG or inhA promoter) and mutant probes (katG or inhA promoter), resistance may be reported as negative (R-), detected (R-det), indeterminate (indet) or undetected (below limit of detection). Additionally, detection of specific INH resistance-associated mutations can result in low-level (INH low R) and high-level (INH high R) resistance results. A resistance negative result suggests a lack of detection of targeted resistance-associated mutations, which may correlate with strain sensitivity, but may not include all potentially resistance-conferring mutations.
- Area 6 - Digital data: Opportunities for integration of diagnostic connectivity solutions and e-systems may be explored to meet targets established in the Framework of indicators and targets for laboratory strengthening under the End TB Strategy (9).
- Area 7 - Quality assurance: Quality assurance systems and activities for the Abbott assays mimic those of other moderate complexity automated NAATs. New method validation for the RealTime MTB RIF/INH test should include precision and accuracy measurement for drugs targeting all drug-resistance loci, using well-characterized strains with and without known resistance-associated mutations. Because of potential contamination of the molecular workflow, each run on the m2000rt instrument must include positive and negative controls to ensure run validity, and laboratory spaces should be tested for contamination at least monthly, using the procedure outlined in the manufacturer's instructions for use.
- Area 9 - Training and competency assessment: Training on the Abbott m2000 system was reported by the Foundation for Innovative Diagnostics (FIND) as being more complex than on other centralized TB testing platforms, requiring theoretical and practical training for at least 5 days (10).
It may be necessary to emphasize training for Realtime assay reagent management and handling, because these reagents are stored under different conditions, and the DNA extraction and PCR reagents require reconstitution and must be manually inserted into the correct positions in the m2000sp instrument. These steps within the procedure can be time consuming and must be done accurately for successful testing runs.
References for A2.1
- Wang MG, Xue M, Wu SQ, Zhang MM, Wang Y, Liu Q et al. Abbott RealTime MTB and MTB RIF/ INH assays for the diagnosis of tuberculosis and rifampicin/isoniazid resistance. Infect Genet Evol. 2019;71:54-9 (https://pubmed.ncbi.nlm.nih.gov/30902741/).
- Update on the use of nucleic acid amplification tests to detect TB and drug-resistant TB: rapid communication. Geneva: World Health Organization; 2021 (https://www.who.int/publications/i/item/update-on-the-use-of-nucleic-acid-amplification-tests-to-detect-tb-and-drug-resistant-tb-rapid-communication).
- Unitaid. Tuberculosis - diagnostics technology landscape, 5th edition. Geneva: World Health Organization; 2017 (https://unitaid.eu/assets/2017-Unitaid-TB-Diagnostics-Technology-Landscape.pdf).
- Abbott RealTime RIF/INH [website]. Chicago: Abbott; 2019 (https://www.molecular.abbott/int/en/products/infectious-disease/realtime-mtb-rif-inh-resistance).
- Abbott RealTime MTB [website]. Chicago: Abbott; 2019 (https://www.molecular.abbott/int/en/products/infectious-disease/realtime-mtb).
- WHO consolidated guidelines on tuberculosis Module 3: diagnosis - rapid diagnostics for tuberculosis detection. Geneva: World Health Organization; 2021 update (https://apps.who.int/iris/bitstream/handle/10665/342331/9789240029415-eng.pdf).
- Evaluation of centralized assays for TB detection and detection of resistance to rifampicin and isoniazid: WHO Technical Expert Consultation Report. Geneva: World Health Organization; 2019 (https://www.who.int/publications/i/item/WHO-CDS-TB-2019.14).
- Considerations for adoption and use of multi-disease testing devices in integrated laboratory network (WHO/HTM/TB/2017.05). Geneva: World Health Organization; 2017 (https://www.who.int/publications/i/item/WHO-HTM-TB-2017.06).
- Framework of indicators and targets for laboratory strengthening under the End TB Strategy (WHO/ HTM/TB/2016.18). Geneva: World Health Organization; 2016 (https://www.who.int/publications/i/item/9789241511438).
- FIND cDST WHO supplement. Geneva: World Health Organization; 2019 (https://www.finddx.org/ wp-content/uploads/2019/08/FIND_cDST_WHO_Supplement.xlsx).
DNA: deoxyribonucleic acid; IR: inactivation reagent; MTBC: Mycobacterium tuberculosis complex; PCR: polymerase chain reaction.
 Feedback
Feedback