Book traversal links for 4.2.1 Decision pathway for Algorithm 2 – LF-LAM testing to aid in the diagnosis of TB among PLHIV
General considerations for Algorithm 2a and Algorithm 2b
- The LF-LAM is a point-of-care test that may be implemented outside the laboratory (e.g. at the bedside in clinics that see critically ill PLHIV) for rapid diagnosis of TB and treatmentinitiation.
- The algorithms may be used for PLHIV being evaluated for pulmonary or extrapulmonary TB.
- The algorithms are appropriate for all PLHIV who meet the testing requirements, regardless of the overall prevalence of HIV in the setting.
- The algorithms are appropriate for both low and high MDR-TB or Hr-TB burden settings. The choice of which mWRD to use may be different in a low- or high-burden MDR-TB setting.
- The algorithms emphasize the use of the LF-LAM test in addition to an mWRD in all PLHIV with signs and symptoms of TB, and in:
- inpatient settings, for HIV-positive adults, adolescents and children with advanced HIV disease or who are seriously ill, or PLHIV with a CD4 cell count of less than 200 cells/mm³ , irrespective of signs and symptoms of TB; and
- outpatient settings, for HIV-positive adults, adolescents and children who are seriously ill or PLHIV with a CD4 cell count of less than 100 cells/mm³ , irrespective of signs and symptoms of TB.
- WHO recommends against using LF-LAM to:
- assist in the diagnosis of active TB in HIV-positive adults, adolescents and children without TB symptoms and an unknown CD4 cell count, or a CD4 cell count greater than 100 cells/ mm³ in outpatient settings; and
- assist in the diagnosis of TB in HIV-negative individuals, because of suboptimal sensitivity and specificity in HIV-negative people.
- Anyone who has signs and symptoms of pulmonary TB and who is capable of producing sputum should have at least one specimen submitted for mWRD testing. This also includes children and adolescents living with HIV who are able to provide a sputum sample (see Algorithm 1).
Decision pathway for Algorithm 2a – LF-LAM testing to aid in the diagnosis of TB among PLHIV in inpatient settings
- Evaluate the hospitalized person for TB, determine HIV status and assess the presence of danger signs for being seriously ill. In PLHIV who are not seriously ill, consider measuring CD4 cell counts, to assess eligibility for testing with the LF-LAM.
- Individuals to be evaluated for TB include hospitalized HIV-positive adults, adolescents and children with signs or symptoms suggestive of TB (pulmonary or extrapulmonary) or with a chest X-ray with abnormalities suggestive of TB, or hospitalized people who have advanced HIV disease (AHD), are seriously ill or have CD4 counts of less than 200 cells/ mm3 , regardless of TB signs and symptoms.
- PLHIV include individuals who are HIV-positive, or whose HIV status is unknown but who present with strong clinical evidence of HIV infection, reside in settings where there is a high prevalence of HIV or are members of a group at risk for HIV. For all those with unknown HIV status, perform HIV testing in accordance with national guidelines. For all adults living with HIV/AIDS, regardless of CD4 cell count or clinical stage, recommend ART and consider initiating co-trimoxazole preventive therapy.
- “Seriously ill” is defined as presenting with any one of the following danger signs: respiratory rate >30 per minute, temperature >39 °C, heart rate >120 beats per minute or unable to walk unaided.
- For adults, adolescents and children aged more than 5 years, AHD is defined as CD4 cell count <200 cells/mm³ or WHO stage 3 or 4 event at presentation for care. All children aged under 5 years are considered as having AHD.
- For hospitalized PLHIV being evaluated for TB 2a A , who are positive for signs and symptoms of TB:
- Collect a urine specimen and conduct the LF-LAM and collect a specimen and conduct mWRD testing. If the mWRD is available on site, perform the mWRD testing in parallel to the LF-LAM testing.
- For individuals being evaluated for pulmonary TB, the following samples may be used for the mWRD: induced or expectorated sputum (preferred), bronchoalveolar lavage, gastric lavage or aspirate. Additional sample types suitable for use with Xpert MTB/ RIF and Xpert Ultra include nasopharyngeal aspirate and for Xpert MTB/RIF includes stool samples.
- For individuals being evaluated for extrapulmonary TB, the Xpert MTB test is recommended for use with CSF (preferred sample for TB meningitis), lymph node aspirates and lymph node biopsies. In addition, Xpert MTB/RIF is recommended for pleural fluid, peritoneal fluid, pericardial fluid, synovial fluid or urine as an initial diagnostic test for the corresponding extrapulmonary TB. Blood may also be used as a specimen for Xpert MTB/RIF for HIV-positive adults and children with signs and symptoms of disseminated TB. Other tests for use in extrapulmonary TB include the Xpert Ultra.
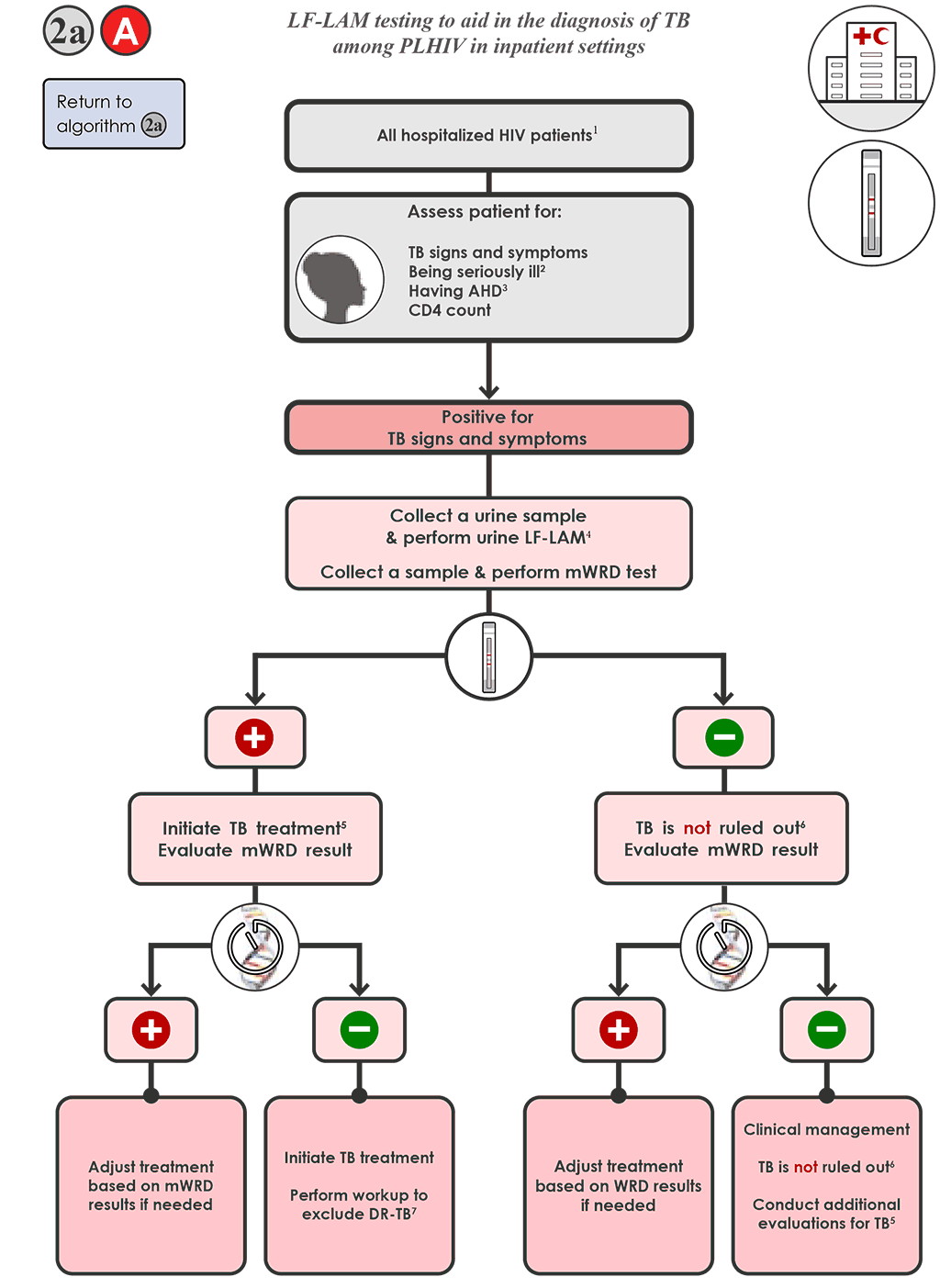
- The LF-LAM result (test time <15 minutes) is likely to be available before the mWRD result and should be interpreted in the context of clinical judgement, chest X-ray findings (if available) and any available bacteriological results.
- All people with TB meeting the testing requirements who have a positive LF-LAM result should be initiated on TB treatment immediately, while awaiting results of the mWRD. Follow Algorithm 1 for the selection and interpretation of mWRD results, which include resistance detection, and modify therapy as needed.
- TB is not ruled out if the LF-LAM test result is negative. Evaluate the results of the mWRD, and follow Algorithm 1 for interpretation of results and follow-up testing.
- Treat all people with TB with an mWRD result of “MTBC detected” for TB (see Algorithm 1), regardless of LF-LAM result.
- TB is not ruled out if both the LF-LAM result and mWRD results are negative (or if no mWRD is performed). Re-evaluate the person and conduct additional testing in accordance with national guidelines. Further investigations for TB may include chest X-ray, additional clinical assessments or culture. Conduct additional clinical evaluations for TB, such as initiating treatment for bacterial infections using antibiotics with broad-spectrum antibacterial activity (but do not use FQs). Consider treatment for Pneumocystis pneumonia. Evaluate clinical response after 3–5 days of treatment.
- If there is clinical worsening or no improvement after 3–5 days of treatment, initiate further investigations for TB and other diseases and, if the person is seriously ill with danger signs, start presumptive TB treatment.
- If there is clinical improvement, reassess for TB and other HIV-related diseases.
- Consider that clinical improvement may occur if the person has TB and a bacterial infection (i.e. clinical improvement does not necessarily rule out TB).
- If there is high clinical suspicion of TB (i.e. clinical history and physical exam, history of previous TB that can be reactivated and chest X-ray suggestive of TB), use clinical judgement as to whether to initiate TB treatment.
- All people with TB should complete the course of treatment for bacterial or Pneumocystis infections.
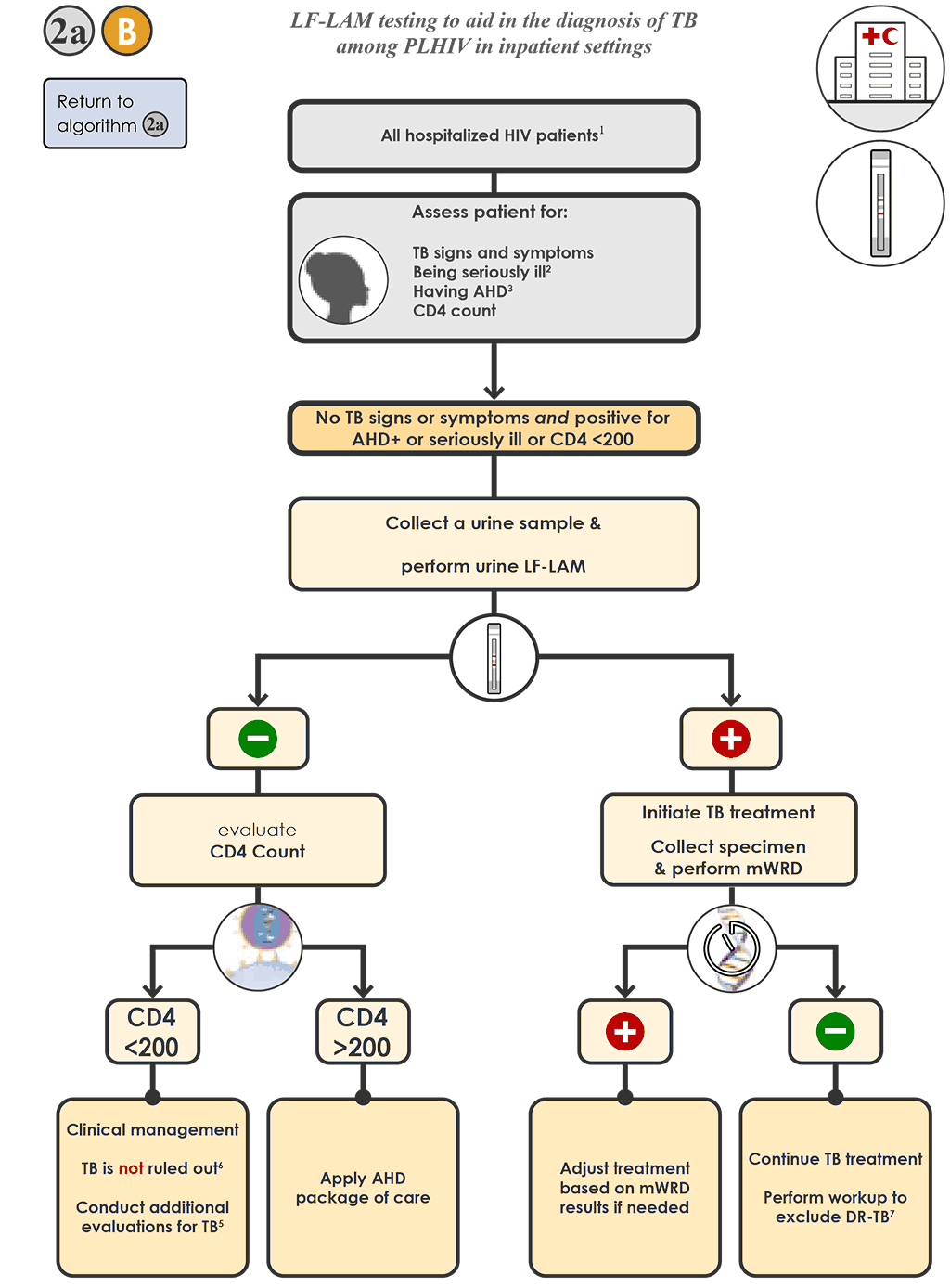
- For hospitalized PLHIV being evaluated for TB who do not have signs or symptoms of TB but have AHD or are seriously ill or have CD4 <200 cells/mm³ 2a B :
- Collect a urine specimen and conduct the LF-LAM.
- If the LF-LAM is negative and the CD4 count is <200 cells/mm³ , re-evaluate the person and conduct additional testing in accordance with national guidelines (see Step 2f).
- If the LF-LAM is negative and the CD4 count is >200 cells/mm³ , apply an AHD package of care.
- If the LF-LAM is positive, initiate TB treatment based on this result and clinical judgement. Collect a specimen and conduct an mWRD to assess the possibility of rifampicin resistance.
- If the mWRD result is “MTBC detected”, follow Algorithm 1 for interpretation, testing and treatment recommendations.
- If the mWRD result is “MTBC not detected”, continue treating the person for TB and conduct additional laboratory testing (e.g. culture and phenotypic DST) to assess drug resistance.
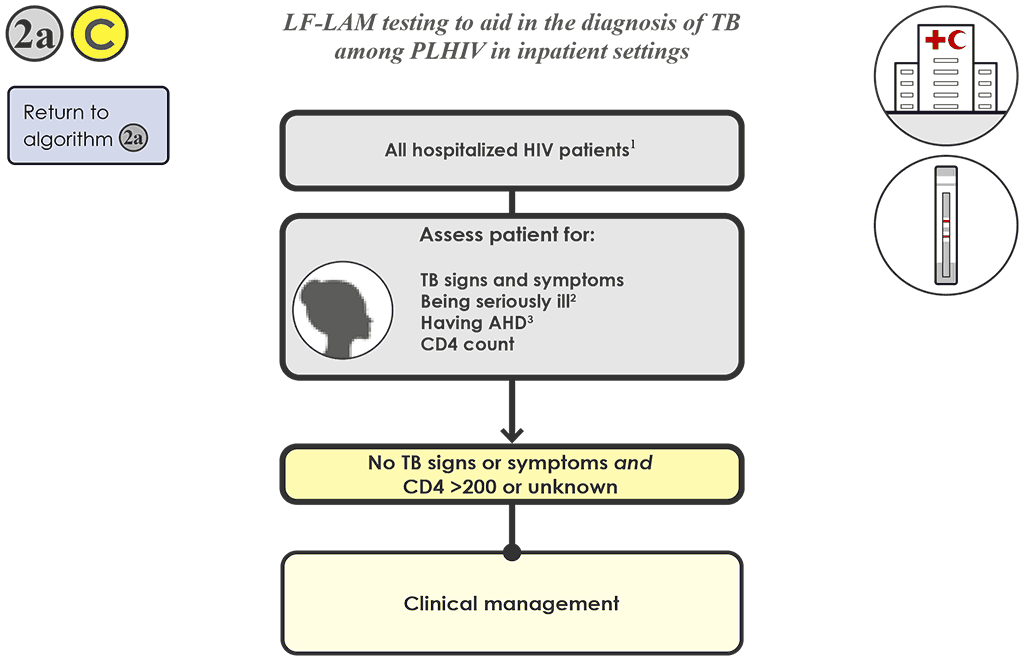
For hospitalized PLHIV without signs or symptoms of TB and whose CD4 is 200 cells/mm³ or above (or is unknown), 2a C do not conduct an LF-LAM test. The latest WHO TB screening guidelines, however, recommend that all hospitalized PLHIV irrespective of signs and symptoms should be routinely tested using an mWRD for TB.
Decision pathway for Algorithm 2b – LF-LAM testing to aid in the diagnosis of TB among PLHIV in clinic and outpatient settings
- Evaluate the person for TB, determine HIV status, and assess the presence of AHD and danger signs for being seriously ill. In PLHIV who are not seriously ill, also consider measuring CD4 cell counts, to assess eligibility for testing with the LF-LAM:
- Individuals to be evaluated for TB include HIV-positive adults, adolescents and children, including all people with TB who are newly diagnosed with HIV and are ART naive, PLHIV returning for care following an interruption of treatment, PLHIV receiving an ART regimen that is failing, and people presenting at the clinic and unwell.
- PLHIV include individuals who are HIV-positive, or whose HIV status is unknown but who present with strong clinical evidence of HIV infection, reside in settings where there is a high prevalence of HIV or are members of a group at risk for HIV. For all those with unknown HIV status, perform HIV testing in accordance with national guidelines.
- “Seriously” ill is defined as presenting with any one of the following danger signs: respiratory rate >30 per minute, temperature >39 °C, heart rate >120 beats per minute or unable to walk unaided.
- For adults, adolescents and children aged more than 5 years, AHD is defined as CD4 cell count <200 cells/mm³ or WHO stage 3 or 4 event at presentation for care. All children aged under 5 years are considered as having AHD.
- For PLHIV being evaluated for TB who are positive for signs and symptoms, or who are seriously ill regardless of TB symptoms 2b A :
- Collect a urine specimen and conduct the LF-LAM and collect a specimen and conduct mWRD testing. If the mWRD is available on site, do the mWRD testing in parallel to the LF-LAM testing.
- For individuals being evaluated for pulmonary TB, the following samples may be used for the mWRD: induced or expectorated sputum (preferred), bronchoalveolar lavage, gastric lavage or aspirate. Additional sample types suitable for use with Xpert MTB/ RIF and Xpert Ultra include nasopharyngeal aspirate and for Xpert MTB/RIF includes stool samples.
- For individuals being evaluated for extrapulmonary TB, the Xpert MTB test is recommended for use with CSF (preferred sample for TB meningitis), lymph node aspirates, lymph node biopsies, pleural fluid, peritoneal fluid, pericardial fluid, synovial fluid or urine as an initial diagnostic test for the corresponding extrapulmonary TB. Blood may also be used as a specimen for HIV-positive adults and children with signs and symptoms of disseminated TB. Other tests for use in extrapulmonary TB include the Xpert Ultra.
 Feedback
Feedback