Book traversal links for Annex 4. Conflict of interest assessment
The DOI forms submitted by the experts reviewing this handbook and information retrieved from the internet, were examined by WHO staff members Nazir Ismail and Carl-Michael Nathanson to assess whether there were, or might be, actual or perceived conflicts of interest and, if so, whether a management plan was required. This evaluation process, and resultant management plans, were based on the Guidelines for declaration of interests (WHO experts) and the WHO handbook for guideline development (2nd edition).
Both financial and non-financial interests were considered. A “significant” conflict of interest would include:
- “intellectual bias”,where an individual may have repeatedly and publicly taken a position on an issue under review, which may affect the individual’s objectivity and independence in the global policy development process;
- involvement in research or publication of materials related to issues under review; and
- a financial interest above US$ 5000.
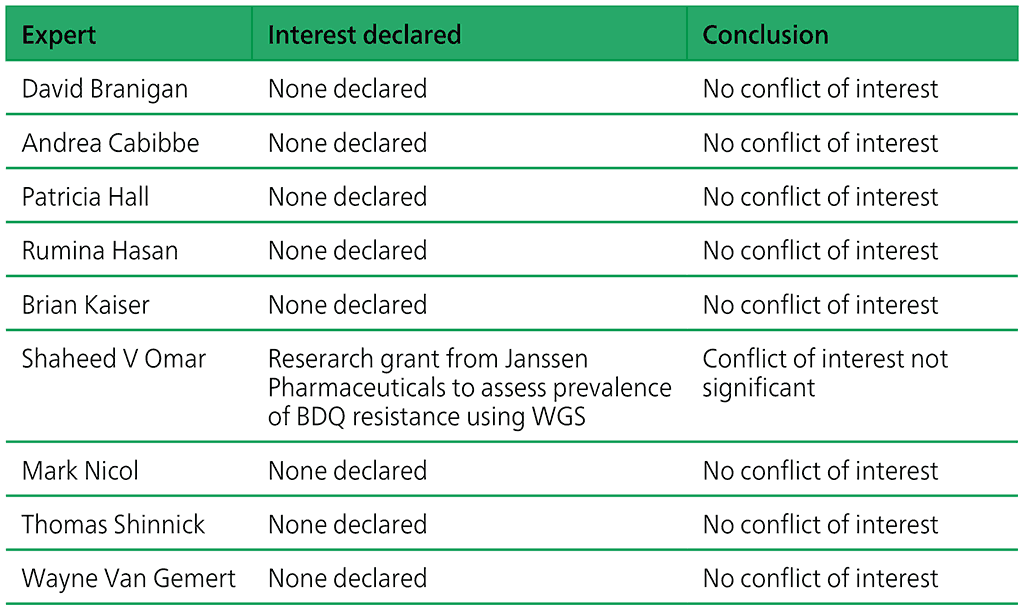
Developers of any assay are never involved in the process of policy development – such involvement is automatically considered a conflict of interest. Upon review no significant conflict of interest were identified. The review findings are summarized in Table A2.1.
Table A2.1. Declarations of interests
 Feedback
Feedback