Book traversal links for 7.2 Composition and duration of the regimen
The duration of Hr-TB treatment is driven by the need to complete 6 months of a fluoroquinolonecontaining regimen. This implies that, when Hr-TB is diagnosed after the start of the regimen for treatment of DS-TB, the companion medicines (HRZE) would end up being given for more than 6 months.
In patients with cavitary disease and with persistent positivity on sputum smear and culture, prolongation of (H)RZE-Lfx beyond 6 months could be considered on a case-by-case basis. Prolongation of treatment increases the risk of toxicity, particularly from pyrazinamide and ethambutol, which are usually only given for 2 months in the first-line TB regimen. The evidence reviewed for the WHO guidance on Hr-TB precluded a recommendation to limit the pyrazinamide duration to less than 4 months when a fluoroquinolone is given.
Levofloxacin is the preferred fluoroquinolone for Hr-TB regimens. The exposure to moxifloxacin decreases markedly when it is combined with rifampicin (116). This effect has not been reported in the case of levofloxacin; also, levofloxacin appears to cause less QT interval prolongation than moxifloxacin (49, 117, 118).
Levofloxacin is included in Hr-TB regimens except in the following instances: when rifampicin resistance cannot be tested for, when there is documented resistance or known intolerance to fluoroquinolones, and when there is pre-existing prolongation of the QT interval and pregnancy. If a fluoroquinolone cannot be used, a patient with Hr-TB can still be treated with 6(H)RZE; streptomycin is not required in such cases.
For patient convenience and ease of administration, the HRZE FDC may be used to treat Hr-TB (given that no RZE FDC is currently available). The dosage of other TB medicines in the Hr-TB regimen is the same as in the standardized DS-TB 2HRZE/4HR regimen. The inclusion of isoniazid in the regimen has not been shown to lead to substantial benefit or harm to patients; however, isoniazid may increase the hepatotoxicity of pyrazinamide (119, 120). High-dose isoniazid (10–15 mg/kg per day) may still be effective when used in combination regimens in the presence of isolated inhA mutations linked to low MIC, even in “fast acetylators” (i.e. individuals who metabolize isoniazid rapidly) (121). In the presence of both inhA and katG mutations, addition of isoniazid (even at a high dose) is unlikely to add value to the regimen.
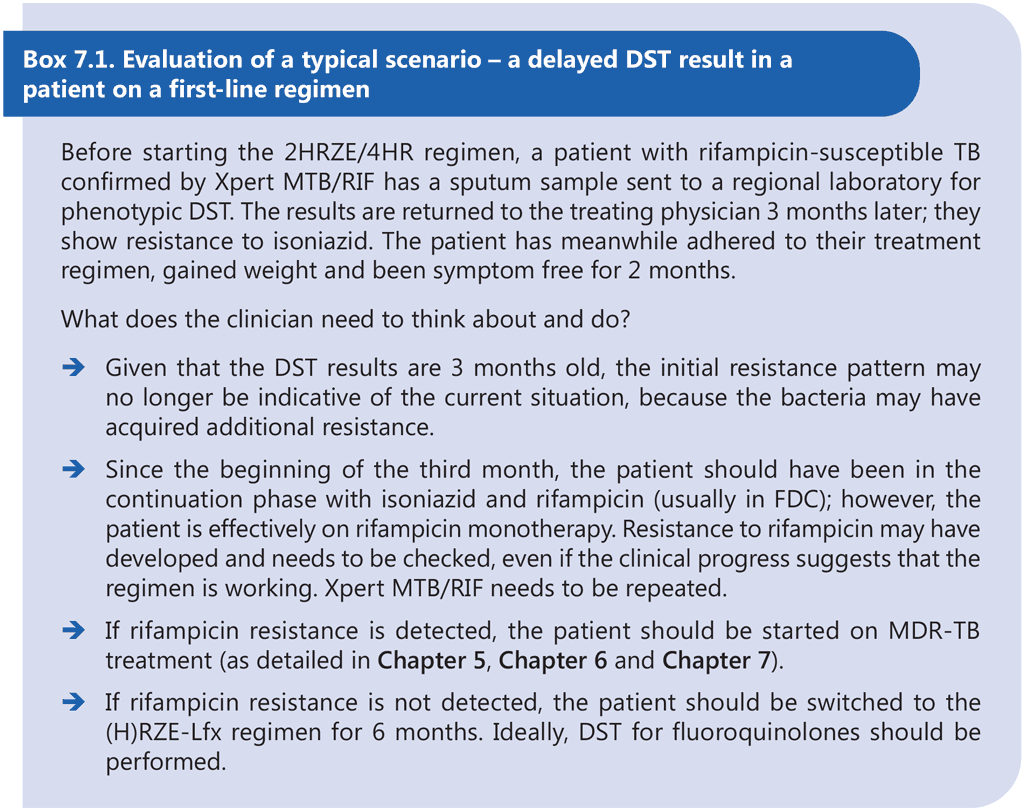
Patients with Hr-TB may have a higher risk of acquiring additional resistance and MDR-TB, which may manifest during the same treatment episode or in a subsequent relapse. The effect of additional resistance to ethambutol and pyrazinamide on the treatment of Hr-TB is unclear.
 Feedback
Feedback