Book traversal links for 2. Diagnostic tests with WHO recommendations
This section provides brief descriptions of WHO-recommended technologies for the detection of TB and DR-TB, summarizes WHO recommendations for such technologies and refers to the WHO consolidated guidelines on tuberculosis Module 3: Diagnosis – rapid diagnostics for tuberculosis detection (7) for a thorough discussion of the technologies and recommendations.
The guidelines updated in 2021 (7) added three new classes of NAAT technologies, as shown in Table 2.1. The most recent update added a fourth new class of technology – targeted NGS.
Table 2.1. Classes of technologies and associated products included in current guidelines
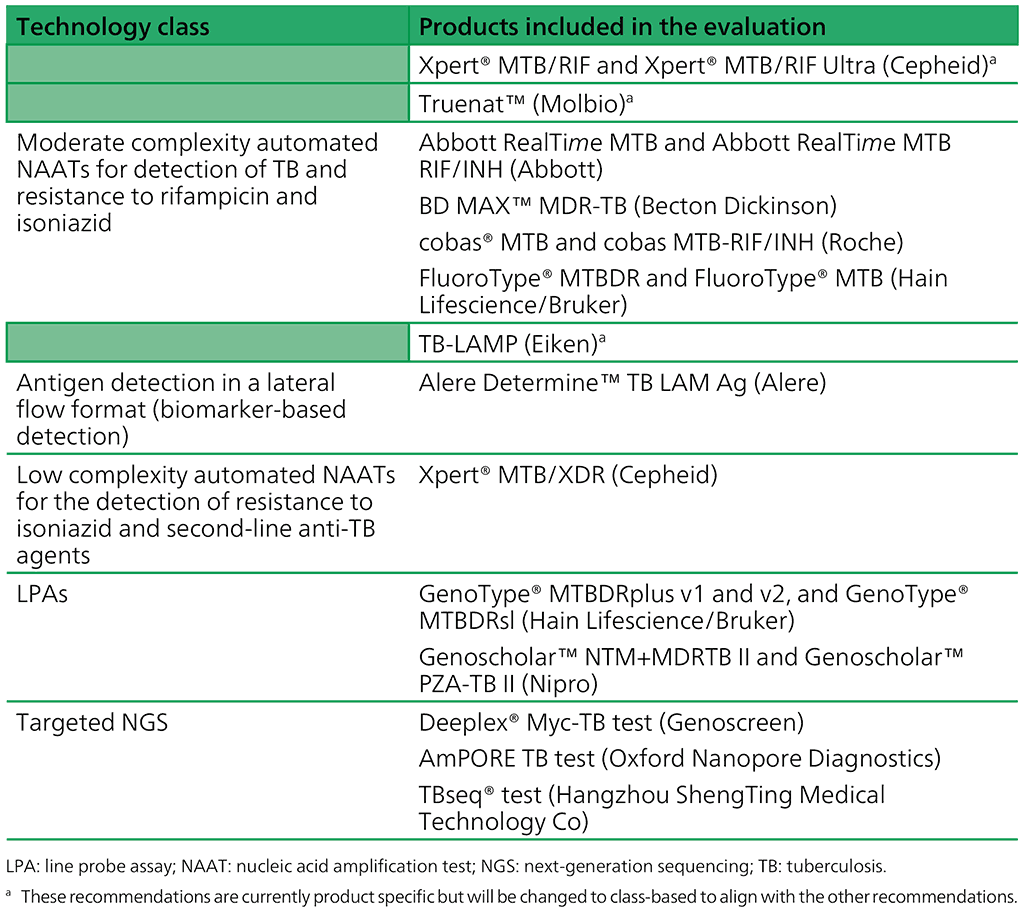
The change from product-specific recommendations (e.g. Xpert MTB/RIF or Truenat MTB) to class-based recommendations (e.g. low complexity automated NAATs) was introduced in 2020. We are currently in a transitionary period, during which the previous product-based recommendations will be integrated into the new classes.
The WHO-recommended tests have also been reorganized in this document to clearly delineate their intended use, as per the recommendations. The new organizational structure is as follows:
- initial tests for diagnosis of TB:
- with drug-resistance detection;
- without drug-resistance detection; and
- follow-on diagnostic tests after TB confirmation.
The initial tests for the diagnosis of TB are broadly grouped as WRDs; these are defined as diagnostic tests that employ molecular- or biomarker-based techniques for the diagnosis of TB (15). The newer, rapid and sensitive molecular tests recommended for the initial detection of MTBC and drug resistance are designated as mWRDs; they include Xpert MTB/RIF Ultra and Xpert MTB/RIF (Cepheid, Sunnyvale, United States of America [USA]), Truenat MTB, MTB Plus and MTB-RIF Dx tests (Molbio Diagnostics, Goa, India) and loop-mediated isothermal amplification (TB-LAMP; Eiken Chemical, Tokyo, Japan).
Also included as mWRDs are the new class of NAATs; that is, the automated moderate complexity NAATs, which detect not only MTBC and RIF resistance but also INH resistance. The four products evaluated and included in this class are Abbott RealTime MTB and MTB RIF/INH assays (Abbott Laboratories, Abbott Park, USA), the BD MAX MDR-TB assay (Becton, Dickinson and Company, Franklin Lakes, USA), the Hain FluoroType MTBDR assay (Bruker/Hain Lifescience, Nehren, Germany), and the Roche cobas MTB and MTB-RIF/INH assays (Hoffmann-La Roche, Basel, Switzerland).
In addition, the biomarker-based lateral flow lipoarabinomannan assay (LF-LAM) test (Alere Determine TB LAM Ag, USA) is recommended to assist in diagnosing TB in selected groups of people living with HIV/AIDS (PLHIV) presumed to also have TB. A positive LF-LAM result is considered to be bacteriological confirmation of TB in these people (15), and this test is also included as a WRD. A negative result does not rule out TB; therefore, LF-LAM should be implemented in parallel with mWRD testing among PLHIV.
WHO has reviewed and recommended each of these tests, and has developed recommendations for their use. In all settings, WHO recommends that rapid techniques be used as the initial diagnostic test to detect MTBC and RIF resistance, to minimize delays in starting appropriate treatment. Follow-on testing for resistance to INH and FQ is important.
Follow-on tests to detect drug resistance include:
- line probe assays (LPAs) for detection of resistance to RIF and INH (GenoType MTBDRplus, Bruker/Hain Lifescience, Nehren, Germany; NTM+MDRTB Detection Kit, Nipro Corporation, Osaka, Japan), and to FQs and second-line injectable agents (GenoType MTBDRsl, Bruker/ Hain Lifescience, Nehren, Germany);
- low complexity automated NAATs for the detection of resistance to INH, FQs, ETO and AMK (first in class: Xpert MTB/XDR [Cepheid, Sunnyvale, USA]);
- high complexity reverse hybridization NAAT for the detection of resistance to PZA (first in class: Genoscholar PZA-TB II [Nipro Corporation, Osaka, Japan]); and
- targeted NGS tests, which couple amplification of selected genes with NGS technology to detect mutations associated with resistance to many anti-TB medicines with a single test (first in class: Deeplex® Myc-TB [GenoScreen] for RIF, INH, PZA, ethambutol [EMB], FQ, BDQ, LZD, CFZ, AMK and streptomycin [STR]; AmPORE TB [Oxford Nanopore Technologies] for RIF, INH, FQ, LZD, AMK and STR; and TBseq® [ShengTing Biotech] for EMB).
 Feedback
Feedback