Book traversal links for A2.4 Information sheet: Practical considerations for implementation of the Bruker-Hain Lifesciences FluoroType MTB and FluoroType MTBDR
Bruker-Hain Diagnostics has two real-time nucleic acid amplification tests (NAATs), the FluoroType MTB to detect Mycobacterium tuberculosis complex (MTBC) and the FluoroType MTBDR, to detect MTBC, and resistance to rifampicin (RIF) and isoniazid (INH) in tuberculosis (TB). The MTB test (VER 1.0) targets the IS6110 DNA insertion element for MTBC detection, while the MTBDR test (VER 2.0) targets the rpoB gene for detection of MTBC and RIF resistance, and the inhA promoter and katG gene for detection of INH resistance. For DNA extraction, both manual (FluoroLyse) and automated (GenoXtract) options are available. The instruments used for amplification and detection are the Bruker-Hain Diagnostics FluoroCycler 12 and FluoroCycler XT for MTB and MTBDR assays. The World Health Organization (WHO) includes these tests within the class of moderate complexity automated NAATs, and the recommendations below apply to them (4).
WHO recommendations for use
In people with signs and symptoms of pulmonary TB, moderate complexity automated NAATs may be used on respiratory samples for detection of pulmonary TB, RIF resistance and INH resistance, rather than culture and phenotypic drug susceptibility testing (DST). (Conditional recommendation; moderate certainty of evidence for diagnostic accuracy)
There are several subgroups to be considered for this recommendation:
- The recommendation is based on evidence of diagnostic accuracy in respiratory samples of adults with signs and symptoms of pulmonary TB.
- This recommendation applies to people living with HIV (PHLIV) (studies included a varying proportion of such people). Performance on smear-negative samples was reviewed but was only available for TB detection and not for RIF and INH resistance. Data stratified by HIV status were not available.
- This recommendation applies to adolescents and children based on the generalization of data from adults. Indeterminate results are more likely to be found with paucibacillary TB disease in children.
- Extrapolation for use in people with extrapulmonary TB and testing on non-sputum samples was not considered because data on diagnostic accuracy of technologies in the class for non-sputum samples were limited.
Key performance conclusions (5, 6)
- Both FluoroType MTB and FluoroType MTBDR assays perform well for the diagnosis of TB, RIF and INH detection compared with culture and phenotypic DST.
- The limit of detection reported by the company for TB detection with FluoroLyse: FluoroType MTB (Version 1.0) = 15 colony forming units (CFU)/mL, FluoroType MTBDR (Version 2) = 9 CFU/mL, while for RIF/INH resistance detection = 14 CFU/mL).
- The pooled sensitivity and specificity data for the class are presented in a Web Appendix of the WHO consolidated guidelines (4).
Test procedure at-a-glance
The FluoroType MTB and MTBDR assays accommodate manual (FluoroLyse kit) or automated DNA extraction (GenoXtract (12) or GenoXtract 96/fleXT instruments with corresponding extraction kits), followed by polymerase chain reaction (PCR) on the FluoroCycler 12 (MTB VER 1.0) or FluoroCycler XT (MTB VER 2.0) instruments. The MTB (VER 1.0) assay uses high resolution melt analysis to detect and automatically report fluorescence detection associated with probes specific for the MTBC insertion element IS6110. The MTBDR assay LiquidArray technology combines sensitive amplification with high resolution melt curve analysis lights-on/ lights-off chemistry to simultaneously detect MTBC and resistance to RIF and INH; it targets the rpoB gene associated with RIF resistance, and the katG gene and the inhA promoter associated with INH resistance. The assay differentiates between high-level and low-level INH resistance, and the FluoroSoftware automatically reports specific mutations identified for each gene target.
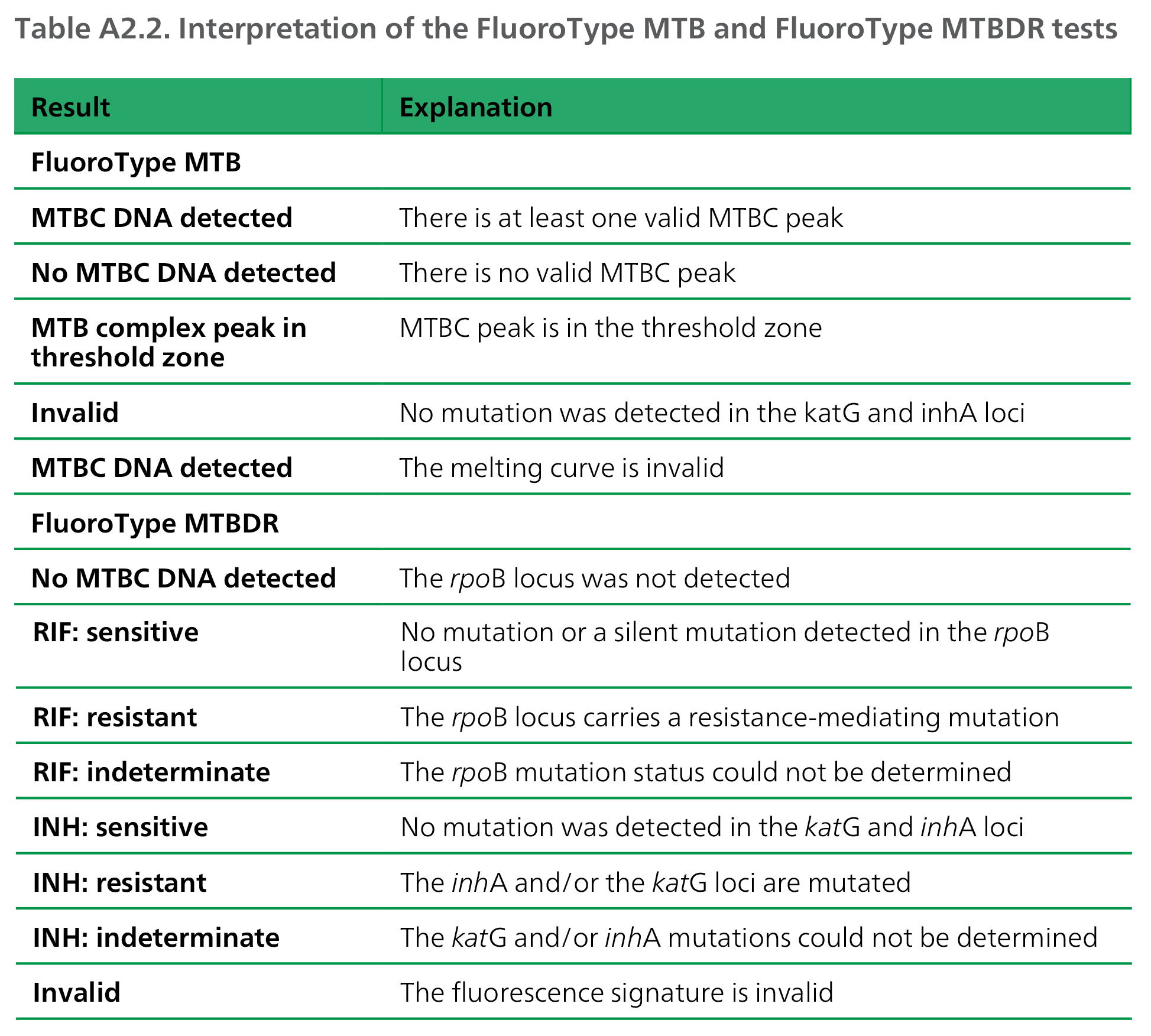
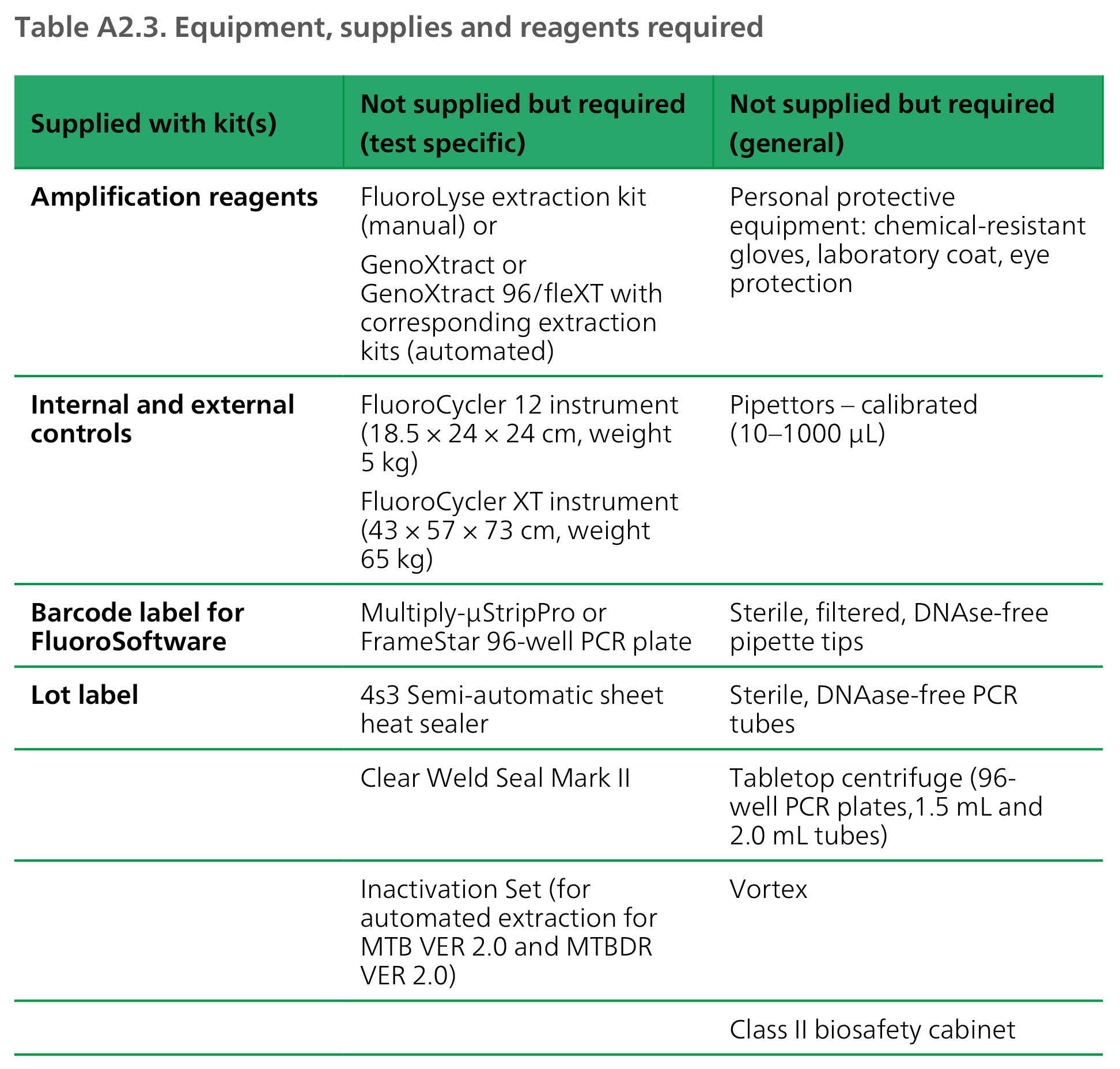
Possible results and their causative explanations are given in Table A2.2, and the equipment, supplies and reagents required are given in Table A2.3.
Operational considerations
- Sample types: Decontaminated sputum specimens (FluoroType MTB), and decontaminated sputum specimens and culture isolates (FluoroType MTBDR).
- Storage and handling: Both amplification reagents and controls (internal and positive MTB) should be stored at -20 °C to -18 °C, refrozen immediately after use and freeze/thawed no more than four times.
- Testing capacity: 12 samples (including assay controls) per FluoroCycler 12 run with potential to for simultaneous connection of multiple units to increase testing capacity. 96 samples (including assay controls) per FluoroCycler XT run.
- Time to detection: Within 3 hours for both FluoroType assays.
- Result reporting: Results are automated via FluoroSoftware, including high-level and low-level INH resistance reporting. Mutations that are rare or are associated with unknown resistance profiles in the target genes are also shown. Additionally, users can share run files or DNA extracts with verified new resistance conferring mutations with the Hain Technical Support Team. These data can be used for machine learning that may improve resistance prediction as new mechanisms of resistance are reported. Note: The rpoB H526C (Escherichia coli nomenclature, equivalent to the H445C M. tuberculosis nomenclature), is not detected by the FluoroType MTBDR.
- Connectivity: Both FluoroCycler instruments can be connected, and data exported, to laboratory information management service.
- Shelf life: As reported on each kit box when stored as directed.
- Unit price: A global price is not yet available through the Stop TB Partnership Global Drug Facility, although discussions are underway.
Implementation considerations
In addition to general guidance provided in Section 3.5, consider the following test-specific implementation considerations:
- Area 1 - Policies and planning: Because of the FluoroCycler and FluoroType tests' high complexity infrastructure and molecular workflow requirements, the FluoroType MTB and MTBDR assays are best suited for centralized reference laboratories (7).
The FluoroCycler instruments on which FluoroType tests are run is capable of multi-disease testing, which may be considered for holistic patient testing and potential cost savings across disease programmes. The following FluoroType assays are available: BK virus, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus, herpes simplex virus, parvovirus B19, Chlamydia trachomatis and Neisseria gonorrhoeae (CT/NGF), methicillin-resistant Staphylococcus aureus (MRSA), Bordetella and Borrelia, sexually transmitted infections (7-plex), severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2) and different human genetics.
• Area 3 - Equipment: Programmes should review existing, local equipment and Bruker-Hain Diagnostics testing protocols, as available, to inform selection and procurement of manual (FluoroLyse) or automated, instrument-based (GenoXtract) extraction resources, noting that the FluoroType tests have not yet been demonstrated as being compatible with existing Hain GenoLyse extraction kits. Similarly, FluoroType MTB and FluoroType MTBDR tests are only compatible with the FluoroCycler instruments and may not be run on any other existing Hain equipment, including the GT Blot system. In addition to obtaining the required instrumentation, testing volumes should be calculated before procurement to maximize resources (human, budgetary and testing) and ensure availability of sufficient testing supplies and reagents to meet clinical demand.
- Area 6 - Digital data: Opportunities for FluoroCycler 12 and FluoroCycler XT integration of diagnostic connectivity solutions and e-systems exists and may be explored to meet targets established in the Framework of indicators and targets for laboratory strengthening under the End TB Strategy (8).
- Area 7 - Quality assurance: Quality assurance systems and activities for the FluoroType assays mimic those of other moderate complexity automated NAATs. Internal and external control reagents are provided and must be included with each run samples to ensure results are accurate and not affected by contamination, amplification inhibition or amplification failure. Control interpretation guidance is included in the manufacturer's instructions for use and should be included in tester trainings and competency assessments. In addition, laboratory spaces should be tested for contamination at least monthly.
- Area 8 - Recording and reporting: The FluoroCycler FluoroSoftware generates automatic reports that include the date, run name and all sample information, as well as the respective fluorescence signatures and the interpretations as derived by the software. Users should follow national requirements for results reporting.
- Area 9 - Training and competency assessment: As with other molecular drug sensitivity tests, laboratory staff and clinicians should be trained on testing principles, methods and the appropriate review and interpretation of results, particularly those related to high and low isoniazid resistance.
References for A2.4
- Unitaid. Tuberculosis - diagnostics technology landscape, 5th edition. Geneva: World Health Organization; 2017 (https://unitaid.eu/assets/2017-Unitaid-TB-Diagnostics-Technology-Landscape.pdf).
- Bruker-Hain Diagnostics. FluoroType MTBDR VER 2.0 - your test system for true MDR-TB testing [website]. (https://www.hain-lifescience.de/en/products/microbiology/mycobacteria/tuberculosis/fluorotype-mtbdr.html).
- Bruker-Hain Diagnostics. FluoroType MTB - the direct detection test for innovative labs [website]. (https://www.hain-lifescience.de/en/products/microbiology/mycobacteria/tuberculosis/fluorotype-mtb.html).
- WHO consolidated guidelines on tuberculosis Module 3: diagnosis - rapid diagnostics for tuberculosis detection. Geneva: World Health Organization; 2021 update (https://apps.who.int/iris/bitstream/handle/10665/342331/9789240029415-eng.pdf).
- de Vos M, Derendinger B, Dolby T, Simpson J, van Helden PD, Rice JE et al. Diagnostic accuracy and utility of FluoroType MTBDR, a new molecular assay for multidrug-resistant tuberculosis. J Clin Microbiol. 2018;56(9)(https://pubmed.ncbi.nlm.nih.gov/29976588/#:~:text=FluoroType%20 identified%2098%25%2C%2097%25,testing%20for%20rifampin%20and%20isoniazid.).
- Update on the use of nucleic acid amplification tests to detect TB and drug-resistant TB: rapid communication. Geneva: World Health Organization; 2021 (https://www.who.int/publications/i/item/update-on-the-use-of-nucleic-acid-amplification-tests-to-detect-tb-and-drug-resistant-tb-rapid-communication).
- Evaluation of centralized assays for TB detection and detection of resistance to rifampicin and isoniazid: WHO Technical Expert Consultation Report. Geneva: World Health Organization; 2019 (https://www.who.int/publications/i/item/WHO-CDS-TB-2019.14).
- Framework of indicators and targets for laboratory strengthening under the End TB Strategy (WHO/ HTM/TB/2016.18). Geneva: World Health Organization; 2016 (https://www.who.int/publications/i/item/9789241511438).
DNA: deoxyribonucleic acid; INH: isoniazid; MTB: Mycobacterium tuberculosis; MTBC: Mycobacterium tuberculosis complex;RIF: rifampicin.
DNA: deoxyribonucleic acid; PCR: polymerase chain reaction; RNA: ribonucleic acid.
 Feedback
Feedback