Book traversal links for 8.5 Chronic liver disease
Isoniazid, rifampicin or pyrazinamide may cause hepatotoxicity. In the management of TB in patients with chronic liver disease (CLD), experts recommend monitoring aminotransferases (i.e. alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) on a weekly basis initially, and fortnightly after the second month of treatment. In cases where aminotransferase are five or more times higher than the upper limit of normal (with or without symptoms), or three or more times higher in the presence of symptoms or jaundice (i.e. bilirubin >3 mg/dL⁻¹), the treatment should immediately be withdrawn. The responsible drugs should be identified, and a sequential reintroduction implemented once enzyme levels have returned to normal. The drug reintroduction should be performed one drug at a time, starting with the drug considered to be the least hepatotoxic, as follows:
- when aminotransferases return to less than two times the upper limit of normal, rifampicin may be restarted with ethambutol;
- after 3–7 days, after checking aminotransferases, isoniazid may be reintroduced, with subsequent rechecking of liver enzymes; and
- if symptoms recur or aminotransferases increase again, the last drug added should be stopped and replaced with another from the list of the recommended drugs (70).
If the clinical pattern indicates cholestasis, rifampicin may be the responsible drug. If the patient has prolonged or severe hepatotoxicity but tolerates isoniazid and rifampicin, a re-challenge with pyrazinamide may be hazardous. In this situation, pyrazinamide may be permanently discontinued, with treatment eventually extended to 9 months (70). In patients with advanced CLD, coagulation factors should be carefully monitored (43, 87-89).
NTPs should consider stocking an extra supply of drugs to modify the HRZE regimen in the treatment of special situations such as CLD. Among the drugs that can be considered safe to use in patients with CLD are ethambutol and fluoroquinolones (70). Given their important bactericidal and sterilizing action, where possible, isoniazid or rifampicin (or both) should be included (70).
A patient’s N-acetyltransferase (NAT) status affects their risk profile. Slow acetylators have a higher possibility of liver injury, so an isoniazid dose of 2.5–5 mg/kg/day may be adequate in such patients; in rapid acetylators, in contrast, the isoniazid dose may be increased to 7.5 mg/kg/day
The Child–Turcotte–Pugh (CTP) score is based on albumin, bilirubin, prothrombin time/international normalized ratio (PT/INR), ascites and encephalopathy. The CTP score can be used as a predictor of tolerance to anti-TB drugs and the treatment outcome, as shown in Table 8.1 (90).
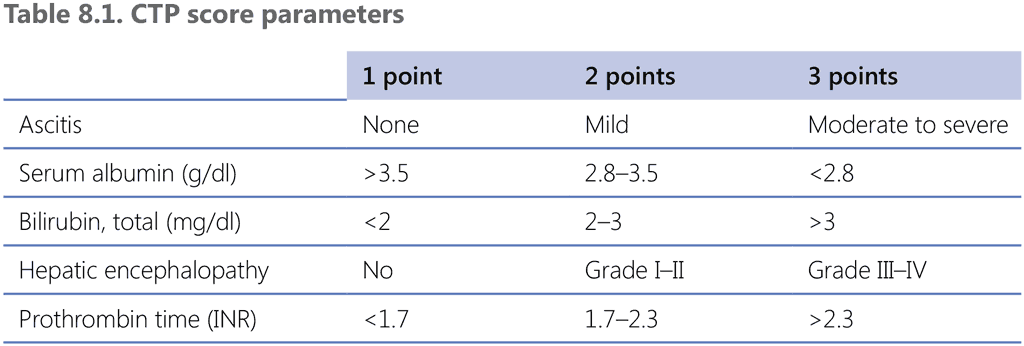
CTP: Child–Turcotte–Pugh; INR: international normalized ratio.

CTP: Child–Turcotte–Pugh.
In people with DS-TB with stable CLD (CTP ≤7), a treatment regimen that includes isoniazid, rifampicin and ethambutol is likely to be tolerated, with the exclusion of pyrazinamide (which is the most hepatotoxic drug in the 6-month regimen). Some experts suggest that, in this situation, the isoniazid and rifampicin continuation phase be prolonged to 7 months, after a 2-month intensive phase with the three drugs (90).
In patients with more severe CLD (CTP 8–10), it is advisable to use only one potentially hepatotoxic drug, preferably rifampicin; however, if CLD is very advanced (CTP ≥11), it is advisable to not use any hepatotoxic drug (70, 85). Some authors advise using a temporary liver-sparing regimen early in treatment to reduce bacillary load and transmission risks while waiting for transaminase levels to decrease.
When there is a need to design regimens for special situations, collaboration with clinicians who have specific experience in CLD and the support of an expert committee (e.g. TB consilium) are recommended (43, 62).
Implementation considerations
- In people with DS-TB and CLD, evaluation of the degree of impairment of the liver function is necessary, to design the best possible regimen that is sufficiently effective while not being aggressive for the liver. Given the clinical severity of these patients, collaboration with clinicians who have specific experience in CLD and the support of an expert committee (e.g. TB consilium) is recommended.
- The NTP should ensure a stock of individual formulations to manage patients with CLD who are unable to tolerate the standard recommended regimens.
- Treatment outcomes are often less favourable in patients with CLD than in patients without CLD.
 Feedback
Feedback