Book traversal links for 3.3.5.1. Implementation considerations
The choice of TPT regimen depends on the age of the child, the HIV and ART status, and the availability and affordability of suitable (child-friendly) formulations.14 Rifampicin- and rifapentine-containing regimens should be prescribed with caution in children and adolescents living with HIV and on ART because of potential drug–drug interactions (see Section 7.1 and Tables 7.2 and 7.3). Table 3.1 summarizes the options for different target and age groups. Note that although 4R is an option, there is no suitable paediatric formulation and therefore this regimen is not included.
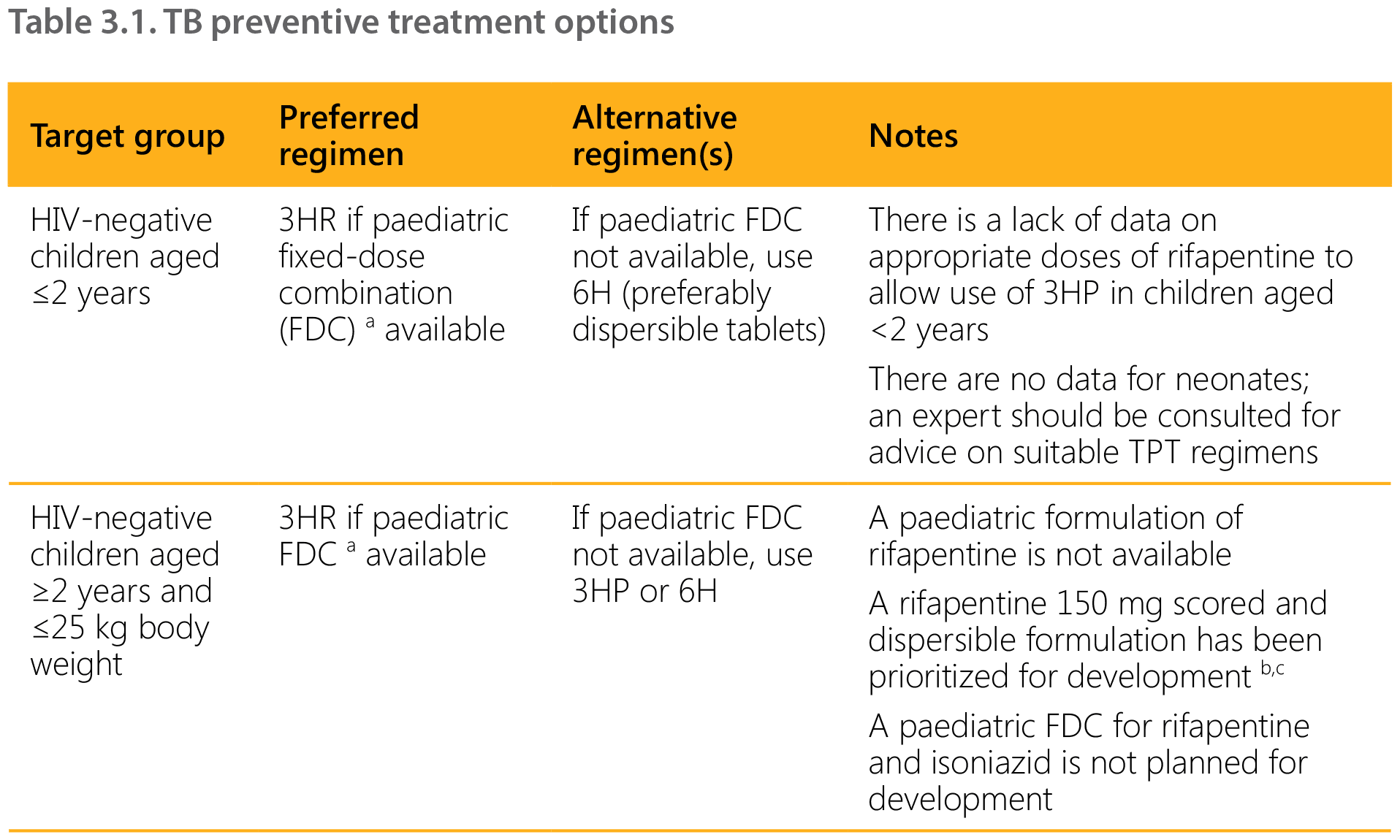
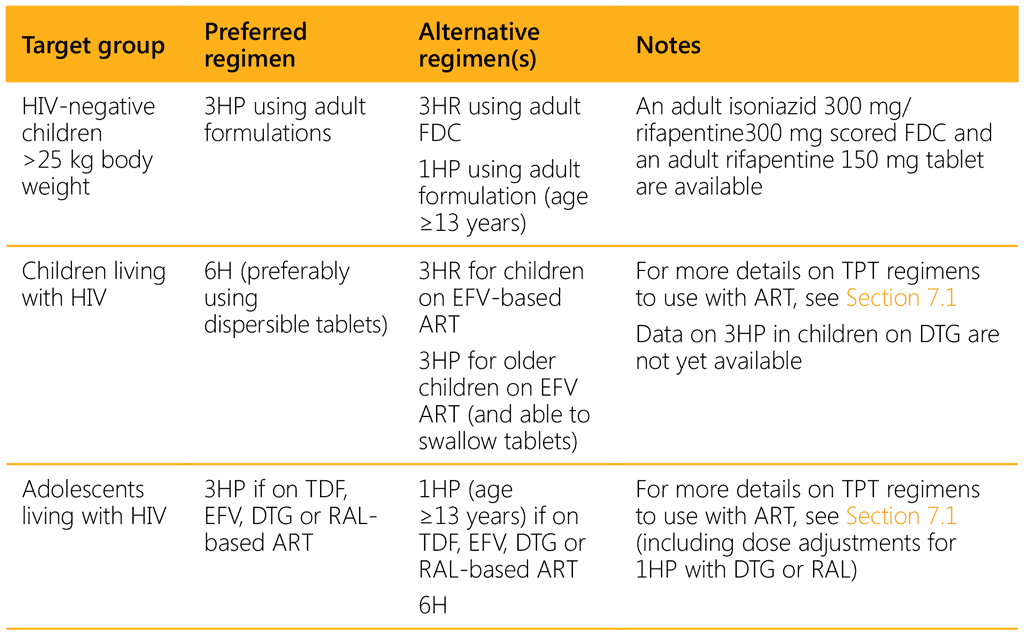
1HP: 1 month of isoniazid and rifapentine daily; 3HP: 3 months of isoniazid and rifapentine weekly; 3HR: 3 months of isoniazid and rifampicin daily; 6H: 6 months of isoniazid daily; DTG: dolutegravir; EFV: efavirenz; FDC: fixed-dose combination; RAL; raltegravir; TDF: tenofovir disoproxil fumarate.
a Dispersible FDC, isoniazid 50 mg/rifampicin 75 mg.
b Report of the meeting to review the Paediatric Antituberculosis Drug Optimization priority list. Geneva: World Health Organization; 2021
(https://www.who.int/publications/i/item/9789240022157).
c Rifapentine 150 mg tablet (scored and dispersible) is included in the 21st Invitation to Manufacturers of Antituberculosis Medicines to Submit an Expression of Interest (EOI) for Product Evaluation to the WHO Prequalification Unit (https://extranet.who.int/pqweb/sites/default/files/documents/EOI_TB_v21_29June2021.pdf).
Infants and young children aged under 5 years are particularly vulnerable due to an increased risk of progressing to TB disease and developing severe forms of TB such as TBM or disseminated TB. In addition, it is often difficult to confirm a diagnosis of TB disease given its paucibacillary nature (63, 64). Averting paediatric TB by delivering TPT is therefore important.
For TPT among children, the 3HR regimen provides a better-tolerated, shorter, more child-friendly option than 6H or 9H, since dispersible FDC formulations are available for young children. A study in four African countries among child contacts starting 3HR demonstrated high uptake and completion rates, and low rates of mild adverse events (65).
As data on an appropriate rifapentine dosage for younger children are lacking, in the short term national programmes could consider scaling up 3HR for TPT among children of all ages (66). Those weighing under 25 kg, including children aged under 2 years, may receive the same formulation used for the continuation phase of TB treatment (2 FDC isoniazid 50 mg/rifampicin 75 mg). Children weighing over 25 kg may receive 3HP (if it is rolled out for adults) or 3HR using adult FDCs of rifampicin and isoniazid. The child-friendly FDC of rifampicin and isoniazid has the added benefit of already being in the national supply chain for the continuation phase of TB treatment for children weighing under 25 kg.
For children living with HIV on lopinavir/ritonavir (LPV/r), dolutegravir (DTG) or nevirapine (NVP) ART, the 6-month isoniazid regimen (6H) is still the most suitable option, as pharmacokinetics and safety studies on rifapentine-based TPT regimens in this population are ongoing. Because of likely drug–drug interactions, due vigilance for signs of isoniazid-induced hepatitis is necessary.
Over the medium to long term, 3HP (or 1HP) may become the preferred regimen across all ages, irrespective of HIV status, provided evidence on an appropriate dose for children aged under 2 years, safety and tolerability is established, and once a dispersible formulation of rifapentine becomes available.
The once-weekly administration, shorter duration of treatment and higher rates of treatment completion with 3HP will likely make it more cost-effective in the long term.
The Paediatric Antituberculosis Drug Optimization Expert Group prioritized a standalone rifapentine formulation that is scored and dispersible, as this will offer the best flexibility for the use of rifapentine for both TPT and possible future first-line TB treatment (66).
14 For available formulations and costs, see the Stop TB Partnership Global Drug Facility Medicines Catalog (http://www.stoptb.org/assets/documents/gdf/drugsupply/GDFMedicinesCatalog.pdf).
 Feedback
Feedback