Book traversal links for 5.2.3. Recommended regimens for treatment of drug susceptible pulmonary TB in children
As in adults, TB treatment in children and adolescents includes a 2-month intensive phase followed by a continuation phase of 2–4 months. In the intensive phase, TB bacilli are rapidly killed to prevent disease progression, transmission and development of drug resistance. In the continuation phase, dormant bacilli are eliminated to effect cure and prevent relapse. The choice of TB treatment regimen (including whether to include a fourth medicine – ethambutol – in the intensive phase) depends on the prevalence of HIV and isoniazid resistance in the setting, severity of disease and age.
In children and adolescents aged between 3 months and 16 years with non-severe TB, a 4-month treatment course is recommended. This recommendation is based on the Shorter Treatment for Minimal Tuberculosis in Children (SHINE) trial, a large phase III trial to evaluate duration of TB treatment in children with non-severe drug-susceptible TB. The trial showed that a 4-month treatment regimen (2 months of isoniazid, rifampicin, pyrazinamide with or without ethambutol, followed by 2 months of isoniazid and rifampicin, 2HRZ(E)/2HR) was non-inferior to the standard 6-month regimen (2 months of isoniazid, rifampicin, pyrazinamide with or without ethambutol, followed by 4 months of isoniazid and rifampicin, 2HRZ(E)/4HR) (86).
In adolescents aged 12 years and over, the 4-month isoniazid, rifapentine, pyrazinamide and moxifloxacin (HPZM) regimen may be used in all settings. This regimen (2HPZM/2HPM) met criteria for non-inferiority for cure of TB in bacteriologically confirmed participants when compared with 2HRZ(E)/4HR in a study that included 63 adolescents. Adverse events were similar in both groups (87).
WHO recommendations on the options to treat children for PTB, including intrathoracic lymph node TB, are summarized in Box 5.2.
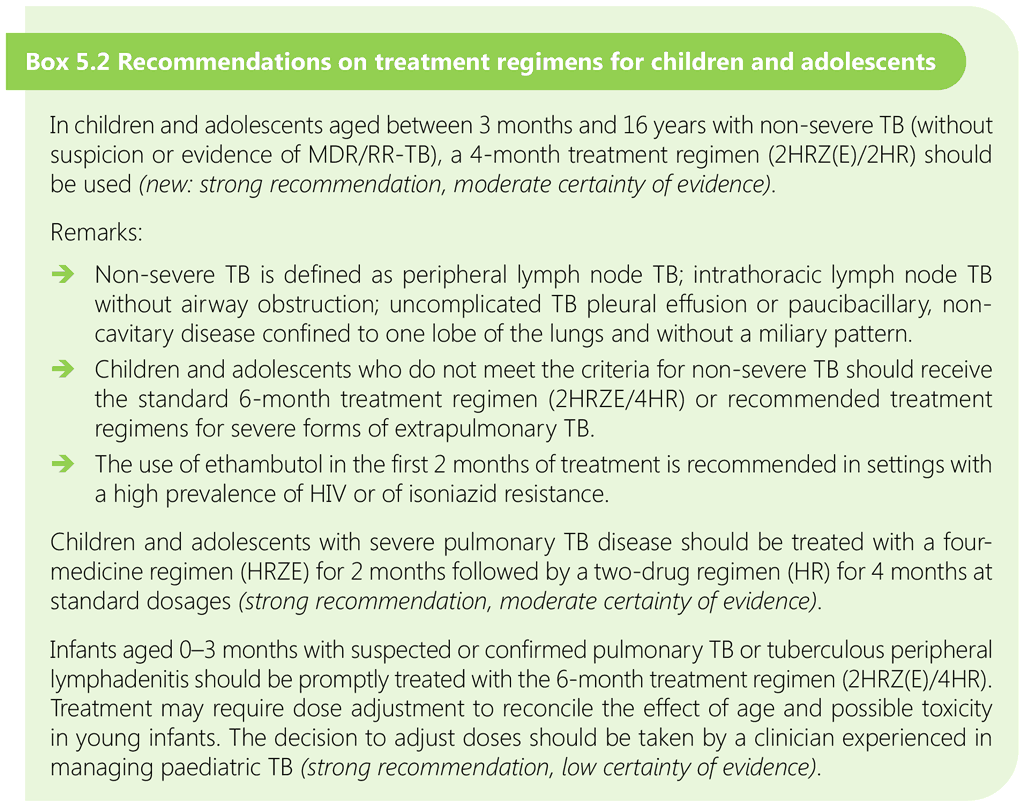

Table 5.1 summarizes regimens for the treatment of PTB by age group, disease severity and local epidemiology.
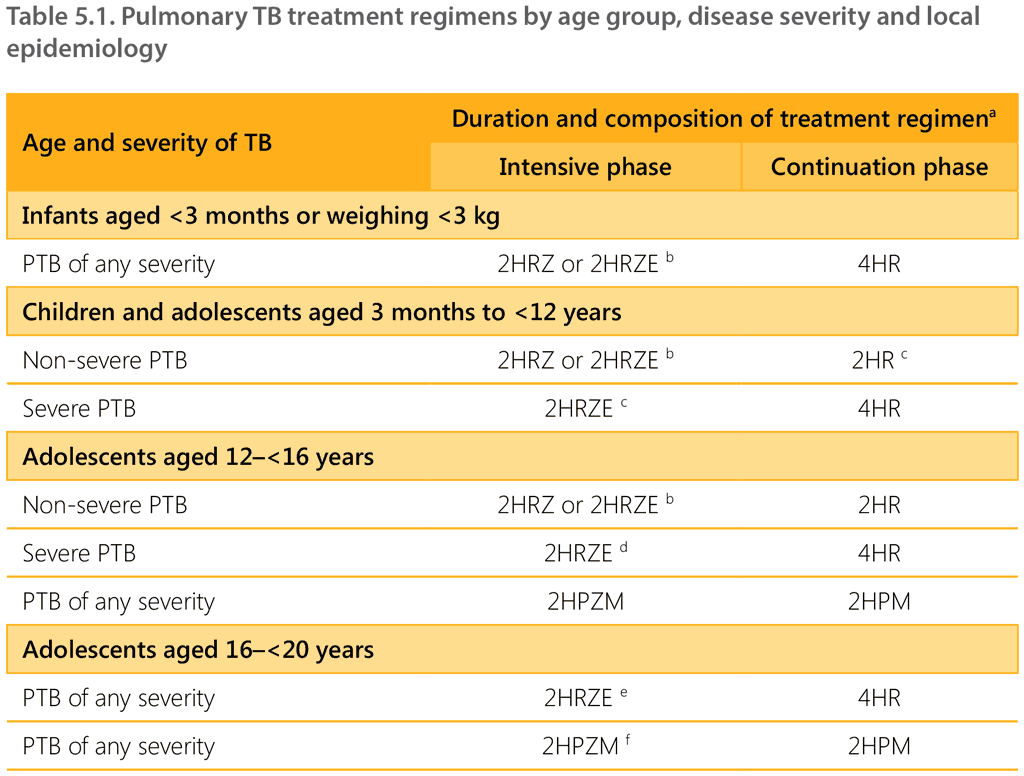
a The standard code for TB treatment regimens uses an abbreviation for each medicine: isoniazid (H), rifampicin (R), pyrazinamide (Z), ethambutol (E), rifapentine (P) and moxifloxacin (M). A regimen consists of two phases – the intensive and continuation phases. The number at the front of each phase represents the duration of that phase in months. For example, 2HRZE consists of treatment with isoniazid, rifampicin, pyrazinamide and ethambutol for 2 months.
b In settings with a high HIV prevalence and/or a high isoniazid resistance prevalence, ethambutol should be added to the intensive phase of treatment. High HIV prevalence settings are defined as HIV prevalence ≥1% among adult pregnant women or ≥5% among people with TB. Thresholds for low, moderate or high levels of isoniazid resistance prevalence are established by country NTPs.
c The SHINE trial was a non-inferiority trial that compared a 4-month regimen (2HRZ(E)/2HR) with a 6-month regimen (2HRZ(E)/4HR). The 4-month regimen was shown to be non-inferior. Therefore, the 6-month regimen may also be used for children with non-severe PTB if the 4-month regimen has not been adopted.
d This regimen applies regardless of HIV prevalence and prevalence of isoniazid resistance.
e This regimen applies to older adolescents regardless of disease severity, HIV prevalence and prevalence of isoniazid resistance.
f This regimen applies to older adolescents regardless of disease severity, HIV prevalence and prevalence of isoniazid resistance, except
for people weighing less than 40 kg and adolescents living with HIV with a CD4 count below 100 cells/mm3.
 Feedback
Feedback