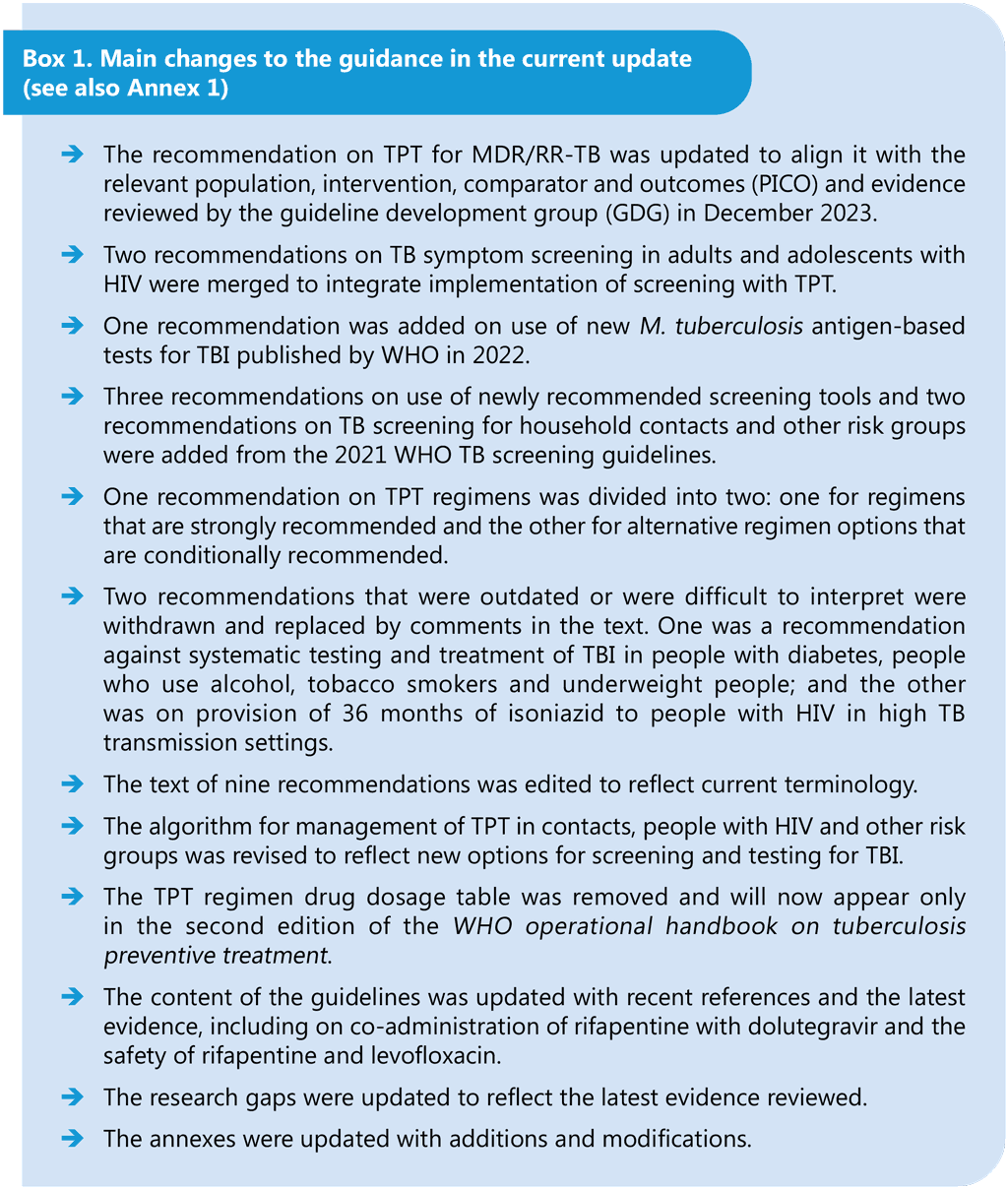
Book traversal links for Executive summary
Tuberculosis infection (TBI) is defined as a state of persistent immune response to stimulation by M. tuberculosis antigens with no evidence of clinically manifest TB disease. It is estimated that about one fourth of the world’s population has been infected with TB. TB preventive treatment (TPT) is one of the key interventions recommended by WHO to achieve the End TB Strategy targets, as upheld by the United Nations High-level Meeting on TB in September 2023. TPT fits within a larger framework of preventive actions envisaged in pillars 1 and 2 of the End TB Strategy, including screening for TB disease, infection control, prevention and care of people with HIV and other co-morbidities and health risks, access to universal health care, social protection and poverty alleviation.
WHO guidelines on TPT account for the probability of progression to TB disease in specific risk groups, the epidemiology and burden of TB and the likelihood of a broad public health benefit of treatment. The main target readership of these guidelines is staff in ministries of health, other policy-makers working on TB, HIV, infectious diseases and maternal and child health and technical partners who support national programmes. This second edition of the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment builds on and supersedes previous WHO guidance on the programmatic management of TB preventive treatment (PMTPT). Its main objectives are to include the latest evidence in its recommendations, particularly on TPT for individuals exposed to multidrug- or rifampicin-resistant TB (MDR/RR-TB) and to update recommendations on systematic TB screening and testing for TB infection (TBI). Some of the text of the recommendations has been revised to improve their clarity (Box 1).
This second edition of the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment was prepared in accordance with the requirements of the WHO Guideline Review Committee. The GDGs considered the certainty of the latest available evidence on effectiveness and harms and of evidence, values and preferences and issues of equity, resource use, acceptability and feasibility of implementation when updating or formulating recommendations and determining their strength. The GDG considered the implications of the best available evidence for each population subgroup at risk, the likelihood of progression from infection to TB disease of each group, and the incidence of TB disease as compared with that in the general population. The GDG used the guiding principle that individual benefit outweighs risk when recommending testing for TBI and TPT. TBI testing is desirable whenever feasible to identify people at highest risk of developing TB. Tools such as chest radiography (CXR) with computer aided detection (CAD) software, C-reactive protein (CRP) and WHO recommended rapid molecular diagnostic tests (mWRD) should be used to rule out TB disease before TPT is started. A requirement for additional resources to implement the guidance should not be viewed as a barrier but should stimulate programmatic mobilization of an appropriate level of funding.
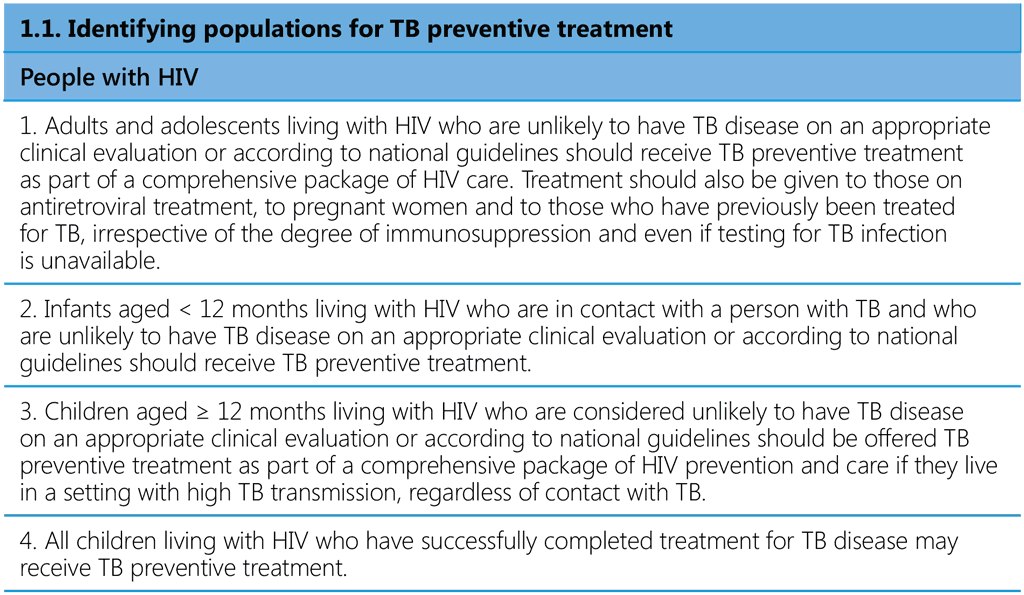
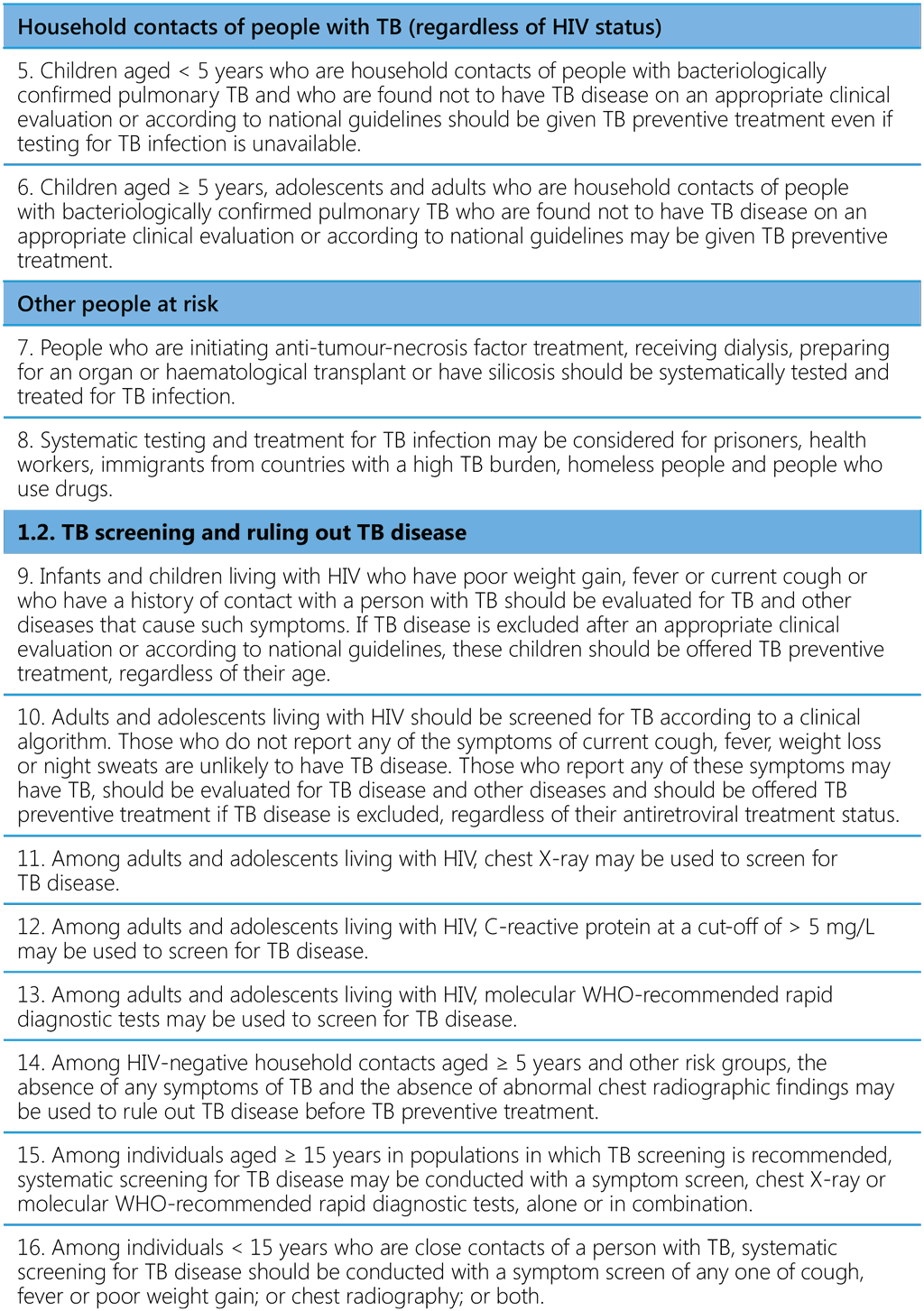
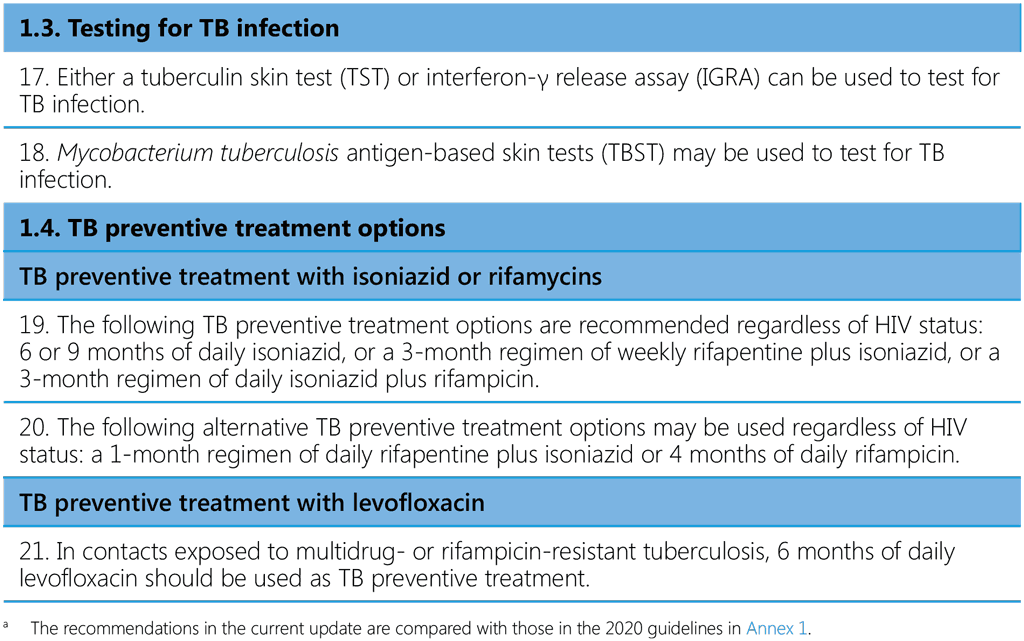
The 21 recommendations in this edition of the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment cover the critical steps in PMTPT and the cascade of preventive care: identification of populations at risk (people with HIV as part of the HIV care package, household contacts and others), TB screening and ruling out TB disease, testing for TBI, providing treatment and support, managing adverse drug reactions and monitoring adverse events, adherence and completion of treatment (Table 1). Most of the recommendations from the 2020 version are largely unchanged. The changes introduced are mainly inclusion of 6 months of daily levofloxacin (6Lfx) as a TPT option for people exposed to MDR/RR-TB in all settings, subject to certain conditions. Other recommendations relevant to PMTPT published in other WHO guidelines since 2020 are included. Operational limitations that require urgent action by countries in order to achieve global targets are highlighted. The new guidelines are accompanied by a second edition of the WHO operational handbook on tuberculosis preventive treatment, which contains practical details on programmatic implementation of the updated guidance. The two publications are being issued as components of the six-module series of WHO consolidated guidelines and operational handbooks, which cover all aspects of TB prevention and care. Both documents will be published on the WHO TB Knowledge Sharing Platform (https://extranet.who.int/tbknowledge).
Table 1. Recommendations in the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment a
 Feedback
Feedback