Book traversal links for Annex 4. Standard operating procedures for sample collection methods
This annex provides examples of standard operating procedures for the most common methods of obtaining clinical samples from children for rapid molecular testing: expectoration, gastric aspiration, nasopharyngeal aspiration (NPA) and sputum induction.
Biosafety and infection prevention and control practices to be followed during cough-inducing and aerosol-generating procedures
Gastric aspiration, NPA and sputum induction are considered aerosol-generating procedures. Although children with TB disease are usually less likely than adults to be infectious, transmission from young children can occur.
The following biosafety considerations are required when performing one of these procedures on a child presenting with predictors of infectiousness (including those with extensive pulmonary or laryngeal involvement, e.g. coughing for more than 3 weeks, cavitary TB disease):
- If the child is considered infectious, these procedures should be performed in a dedicated cough room with directional airflow with 6–12 air exchanges per hour.
- All personnel involved must use personal protective equipment (particulate respirators such as N95 or FFP2 masks, eye protection glasses, gloves and a plastic apron). Caregivers assisting personnel with the procedure (e.g. holding a child during specimen collection) should be provided with particulate respirators.
- Put up a sign outside the room indicating that specimen collection is taking place inside, to avoid anyone entering the room during the procedure.
- Wash hands as per the local handwashing protocol before and after the procedure. All surfaces must be cleaned with 70% alcohol solution before and after each procedure.
- After collection, specimen containers should be closed tightly. The outside of the container should be disinfected before it is labelled. The sample should then be placed in a sample bag.
- After the procedure, masks, gloves and disposable aprons should be discarded in a plastic bag or appropriate bin, and hands should be disinfected immediately.
- Allow at least 10 minutes after specimen collection before exiting the room. Close the door immediately. Clearly indicate the time when the next procedure can be done.
Expectoration30
Background
All sputum specimens produced by children and adolescents should be sent for an mWRD. Children and adolescents who can produce a sputum specimen may be able to transmit the disease. They should be asked to produce the specimen outside and not in an enclosed space (e.g. a bathroom) unless there is a room especially equipped for sputum collection.
Procedure
- Reassure the child or adolescent by explaining to them and their family the reason for sputum collection and the procedure.
- Ask the child or adolescent to rinse their mouth with water before producing the specimen. This will help to remove food and any contaminating bacteria in the mouth.
- Ask the child or adolescent to take two deep breaths, holding the breath for a few seconds after each inhalation and then exhaling slowly. Ask the child or adolescent to breathe in a third time and then forcefully blow the air out. Ask them to breathe in again and then cough. This should produce sputum from deep in the lungs. Ask the child or adolescent to hold the sputum container close to the lips and to spit into it gently after a productive cough.
- If the amount of sputum is insufficient, encourage the child or adolescent to cough again until a satisfactory specimen is obtained. Many people cannot produce sputum from deep in the respiratory tract in only a few minutes. Give the child or adolescent sufficient time to produce an expectoration that they feel is produced by a deep cough.
- If there is no expectoration, treat the container as used and dispose of it in the appropriate manner.
Gastric aspiration31
Background
Children with TB may swallow mucus that contains M. tuberculosis. Gastric aspiration is a technique used to collect gastric contents to try to confirm the diagnosis of TB by rapid molecular tests (or mycobacterial culture if rapid tests are not available). Gastric aspirates are used for collection of samples for diagnostic testing in young children when sputum cannot be spontaneously expectorated or induced using hypertonic saline. It is most useful for young children who are hospitalized. Gastric aspirates are collected from young children with presumed pulmonary TB (PTB). During sleep, the
mucociliary system of the lung beats mucus up into the throat. The mucus is swallowed and remains in the stomach until the stomach empties. The highest-yield specimens are therefore obtained first thing in the morning.
Materials needed
- non-sterile gloves;
- particulate respirator masks (N95 or equivalent);
- disposable apron;
- eye protection glasses;
- nasogastric tubes (6–10 French), preferably Ryles or Levin tubes;
- 5, 10 and 20 mL syringes;
- sterile specimen container with screw top (Falcon tube);
- litmus paper/pH strips;
- sodium bicarbonate 4% solution for bedside neutralization;
- three bedsheets or surgical drapes (one for the bed, one to wrap child, one to cover child);
- dropper or small syringe;
- normal saline (0.9% NaCl) or sterile water in single-use vials;
- local anaesthetic gel;
- optional oxymetazoline (to prevent epistaxis);
- alcohol or chlorhexidine;
- laboratory request form;
- permanent marker or pen;
- antiseptic soap.
Contraindications
- child not fasted for 4 hours (3 hours for infants);
- low platelet count or bleeding tendency;
- obstructive lesions in nasopharyngeal tract.
Procedure
- This procedure is routinely performed by nursing personnel.
- The child’s parent or guardian should be instructed regarding overnight fasting of at least 4 hours before early-morning gastric aspirate. The procedure is preferably performed early in the morning. It may also be performed during the daytime, as long as the child has fasted for a minimum of 4 hours.
- Use an assistant (e.g. caregiver) to help.
- Prepare all equipment before starting the procedure.
- Disinfect all working surfaces, including the bed. Place a drape over the bed. Use a drape to secure the child and another drape to cover the child, leaving their head exposed.
- Position the child on their back or side with the help of an assistant.
- Optional: instil 2 drops of oxymetazoline into each nostril to induce vasoconstriction and prevent epistaxis.
- Measure the distance of the nasogastric tube between the child’s nose and stomach to estimate how far the tube will need to be inserted to reach the stomach.
- Coat the outside of the nasogastric tube with local anaesthetic gel, without covering the holes.
- Place the child’s face in the “sniffing air” position, and then pass the nasogastric tube from the nose into the stomach to aspirate gastric contents.
- Attach a syringe (10 mL if using Levin tube or 20mL if using Ryles tube) to the nasogastric tube (size 6–10 French, depending on the size of the child).
- Withdraw (aspirate) gastric contents using the syringe attached to the nasogastric tube.
- To check the position of the tube is correct, test the gastric contents with litmus paper: blue litmus turns red in response to acidic stomach contents. (This can also be checked by pushing 3–5 mL air from the syringe into the stomach and listening with a stethoscope over the stomach.)
- Aspirate stomach contents gently and steadily, with the child in each of three positions: head central, left lateral and right lateral. Allow a few seconds before aspirating after changing position. If no fluid is aspirated, push the tube 1–2 cm deeper or pull it out 1–2 cm, and then aspirate. Ideally 5 mL should be collected (especially in a sick child), but any volume of more than 1 mL is adequate for bacteriological testing.
- If less than 1 mL is aspirated, a gastric lavage can be performed:
- Insert 10 mL of sterile water or preservative-free normal saline in the nasogastric tube, leave for 3 minutes, and then aspirate until a minimum of 5–10 mL aspirate is obtained.
- If no fluid is aspirated, insert an additional 10 mL of sterile water and aspirate again. If still unsuccessful, repeat this up to three times.
- Transfer the full volume of gastric fluid from the syringe into a sterile container (Falcon tube).
- Titrate 4% sodium bicarbonate using a pipette or syringe and pH strips, adding 0.3 mL aliquots to the specimen until pH 6–7 is reached. (This neutralizes the acidic gastric contents and prevents destruction of TB bacilli.) Check pH after each addition of bicarbonate using litmus paper.
After the procedure
- Clean the Falcon tube with alcohol swabs.
- Label the sample: sample type and number, date, time, time of neutralization, volume of bicarbonate added, and total sample volume.
- Complete the laboratory request forms.
- Transport the specimen in a cool box to the laboratory for processing as soon as possible (within 4 hours).
- If it is likely to take more than 4 hours for the specimens to be transported, place them in the refrigerator (at 4–8 °C) and store until transported.
- Give the child their usual food.
Nasopharyngeal aspiration (NPA)32
Materials needed
- non-sterile gloves;
- particulate respirator masks (N95 or equivalent);
- disposable apron;
- eye protection glasses;
- suction machine (mucus aspirator);
- sterile nasogastric catheter (6–10 French) or mucus extractor (6–8 G);
- normal saline (0.9% NaCl) or sterile water in single-use vials;
- one or two bedsheets to wrap the child;
- optional oxymetazoline (to prevent epistaxis);
- cotton wool;
- alcohol or chlorhexidine;
- sterile sample container (Falcon tubes);
- laboratory request form;
- permanent marker or pen;
- antiseptic soap.
Contraindications
- child not fasted for 2 hours;
- low platelet count or bleeding tendency;
- obstructive lesions in nasopharyngeal tract.
Procedure
- Clearly explain to the child and their family the reason for collecting nasopharyngeal aspirate and the main steps of the procedure.
- Place the child in a supine position on their back or side, or sitting on a family member or caregiver’s lap.
- To avoid injury to the child due to movement, young children should be wrapped in a piece of cloth, and an assistant nurse asked to hold the child’s head throughout the procedure.
- Clean the child’s nose with saline drops and cotton wool. If the child is old enough, ask them to blow their nose into a tissue. If the nasal mucus is too thick to be removed with the measures above, it can be suctioned before NPA. A soft catheter size F6/7 should be used for suctioning and then discarded immediately afterwards.
- Connect the mucus extractor (sputum trap) to the suction pump and the catheter. Do not connect the mucus extractor directly to the suction machine.
- One drop of oxymetazoline may be instilled into each nostril to prevent epistaxis.
- Instil two drops of sterile saline into each nostril.
- Measure the distance from the nostril to the external opening of the ear to find the length of catheter used to aspirate the NPA sample.
- Choose the size of the catheter and adjust the pressure depending on the age of the child:
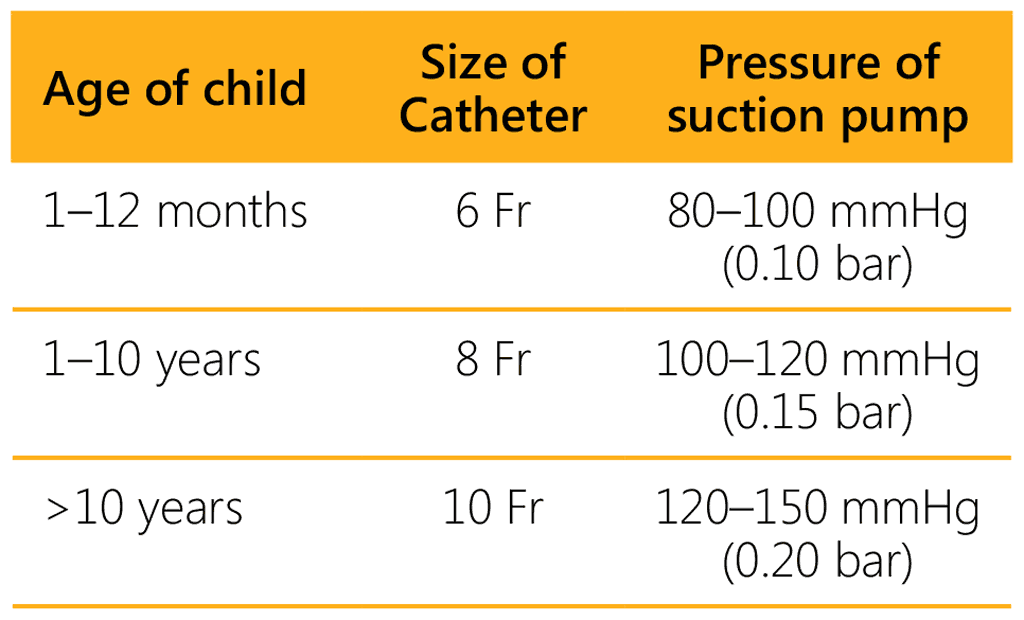
- Without applying suction, insert the tube through the child’s nostril, along the posterior pharyngeal wall until the marked length is reached. If the child does not have teeth, the tube can be introduced via the mouth. Proceed with caution to avoid causing trauma. Usually, the tube makes the child cough and produce sputum, which can then be aspirated.
- Suctioning is activated only when the tip of the catheter is in the posterior nasopharynx.
- Using a rotating movement, collect respiratory secretions by slowly pulling out the tube. Do not push the tube forward while aspirating, as this increases the risk of local trauma.
- The catheter should remain in the nasopharynx for a minimal period of time, and no more than 10 seconds.
- The procedure should aspirate 2–5 mL of secretions. If this volume is not reached with the first aspiration, the procedure should be repeated with nasopharyngeal lavage by inserting 5 mL of normal saline into the same or other nostril.
- The procedure must not be attempted more than three times.
- Stop the procedure immediately if:
- respiratory distress occurs;
- profuse sweating, nausea, vomiting, light-headedness, dizziness or loss of consciousness occurs.
After the procedure
- Monitor the child for several minutes.
- Inform the child’s parent or caregiver that coughing may be more frequent for 24 hours after the procedure.
- Transfer the full volume of sample into a sterile container (Falcon tube).
- Clean the Falcon tube with alcohol swabs.
- Label the sample with sample type and number, date, time and total sample volume.
- Place the specimen in a sample bag, seal and prepare for transport.
Sputum induction33
Background
Sputum induction is typically done in people of any age who are unable to produce sputum spontaneously. The patient inhales nebulized hypertonic saline solution, which liquefies airway secretions, promotes coughing and allows expectoration of respiratory secretions. In young children, inhalation of hypertonic saline does not always trigger expectoration. When expectoration does not occur, NPA is usually required for sputum collection.
Sputum induction is regarded as a low-risk procedure for the child or adolescent to be evaluated for TB. The very few adverse events that have been reported include coughing spells, mild wheezing and nosebleeds. The procedure can be performed safely even in young infants, although staff will need to have specialized training and equipment to perform the procedure in such patients and infection control measures must be observed. Examine children and adolescents in advance to ensure they
are well enough to undergo the procedure.
Children and adolescents with the following characteristics should not undergo sputum induction:
- not fasted for 3 hours;
- oxygen saturation less than 92% in room air or cyanosis;
- severe respiratory distress and vital signs outside normal parameters;
- moderate to severe wheezing;
- severe cough;
- bleeding – low platelet count, nosebleeds or other bleeding source;
- reduced level of consciousness.
Materials needed
- non-sterile gloves;
- particulate respirator Masks (N95 or equivalent);
- disposable apron;
- protective eyeglasses;
- salbutamol (100 μg/puff);
- spacer;
- oxygen concentrator with mask or nasal cannula;
- pulse oximeter;
- nebulizer and tubing;
- nebulization mask of different sizes or mouthpiece;
- antibacterial filter;
- sterile hypertonic saline solution (3–5%);
- 5–10 mL syringes;
- sputum container;
- suction pump;
- sterile nasogastric catheter (6–10 Fr) or mucus extractor (6/7/8 G);
- one or two bedsheets to wrap the child;
- optional oxymetazoline (to prevent epistaxis);
- mucus trap;
- sterile saline solution;
- cotton wool;
- alcohol or chlorhexidine
- disinfectant for medical equipment;
- sterile sample container (Falcon tubes);
- laboratory request form;
- permanent marker or pen;
- antiseptic soap.
Procedure
Sputum induction is performed by a nurse or other clinician trained in the technique and is undertaken after a 2–3 hour fast. General observations and chest auscultation should be documented. Oxygen saturation and pulse rate must be monitored throughout the procedure. The procedure must be stopped in the event of a fall in saturation below 90% and a pulse over 180/minute or below 100/minute.
- Explain the procedure to the child or adolescent and to their parent or caregiver (if present).
- Administer a bronchodilator (e.g. salbutamol 200 μg via a metered dose inhaler with attached spacer) to prevent bronchoconstriction. Wait 15 minutes before starting nebulization.
- Fill the medication chamber cup of the nebulizer with 10 mL of sodium chloride 5% solution.
- Administer nebulized hypertonic saline (5% NaCl) for 15 minutes or until the reservoir is empty.
- Carry out chest physiotherapy (lightly clapping or percussing on the back, chest and underarms of the child using cupped hands) if necessary to mobilize secretions.
- For older children and adolescents who are able to expectorate:
- Encourage the child or adolescent to expectorate sputum into a sputum container. The child or adolescent should continue to expectorate until no more sputum can be produced. Nebulization can be repeated if an inadequate sample is obtained (less than 1 mL or watery sample indicative of saliva).
- If the child or adolescent does not cough after nebulization, encourage them to perform deep breathing or to jump or run on the spot if clinically stable and able to do so. Chest percussion is done over the anterior and posterior chest wall. Encourage the child or adolescent to expectorate as above.
- If less than 1–2 mL of sputum is collected, repeat nebulization with another 5 mL of sodium chloride 5%, until at least 2 mL of sputum is collected, allowing for an interval of at least 30 minutes between the end of one nebulization and the start of the next. No more than three consecutive nebulizations are recommended in one session.
- Seal the specimen container tightly.
- Ensure the child or adolescent is wearing a surgical mask before they leave the sputum induction room.
- For young children who are unable to expectorate:
- Obtain sputum by suctioning through the nasopharynx with a sterile mucus extractor or nasogastric catheter (see procedure for nasopharyngeal aspiration).
Any equipment that will be reused must be disinfected and sterilized before use with subsequent patients.
30 Adapted from Guidance for national tuberculosis programmes on the management of tuberculosis in children, second edition. Geneva: World Health Organization; 2014.
31 Adapted from Guidance for national tuberculosis programmes on the management of tuberculosis in children, second edition. Geneva: World Health Organization; 2014; and Standard operating procedures: collection, transport and processing of gastric aspirates. CaP-TB project.
32 Adapted from Standard operating procedures: collection, transport and processing of nasopharyngeal aspirates. CaP-TB project; and TB-Speed standard operating procedure nasopharygeal aspirate (NPA) collection, TBS_2P_SOP_BSC, version 1.0, 14/02/2019.
33 Adapted from Guidance for national tuberculosis programmes on the management of tuberculosis in children, second edition. Geneva: World Health Organization; 2014; and Standard operating procedures: collection, transport and processing of induced sputum. CaP-TB project.
 Feedback
Feedback