Book traversal links for Loop-mediated isothermal amplification
A commercial molecular assay, the Loopampᵀᴹ Mycobacterium tuberculosis complex (MTBC) detection kit (Eiken Chemical Company, Tokyo, Japan), is based on loop-mediated isothermal amplification (LAMP) reaction. Referred to as TB-LAMP, this is a manual assay that requires less than 1 hour to perform and can be read with the naked eye under UV light. Because it requires little infrastructure and is relatively easy to use, TB-LAMP is being explored for use as a rapid diagnostic test that would be an alternative to smear microscopy in resource-limited settings. LAMP methods have been used to detect malaria and several neglected tropical diseases.
In 2012, WHO convened a GDG on TB-LAMP recognizing that it is a manual molecular test to detect TB and could feasibly be implemented in peripheral-level microscopy laboratories with adequately trained laboratory technicians. The advantages of TB-LAMP are that it has a relatively high throughput, does not require sophisticated instruments, and has biosafety requirements similar to those of sputum-smear microscopy. Since 2012, some 20 additional studies in 17 countries have been conducted. WHO convened a GDG meeting in January 2016 to review evidence from a systematic review and meta-analysis of data from individual participants in these studies.
Recommendations
- TB-LAMP may be used as a replacement test for sputum-smear microscopy for diagnosing pulmonary TB in adults with signs and symptoms consistent with TB.
(Conditional recommendation, very low quality evidence) - TB-LAMP may be used as a follow-on test to smear microscopy in adults with signs and symptoms consistent with pulmonary TB, especially when further testing of sputum smear-negative specimens is necessary.
(Conditional recommendation, very low quality evidence)
Remarks
- These recommendations apply to settings where conventional sputum-smear microscopy can be performed.
- TB-LAMP should not replace the use of rapid molecular tests that detect TB and resistance to rifampicin, especially among populations at risk of MDR-TB.
- The test has limited additional diagnostic value over sputum-smear microscopy for testing PLHIV who have signs and symptoms consistent with TB.
- These recommendations apply only to the use of TB-LAMP in testing sputum specimens from patients with signs and symptoms consistent with pulmonary TB.
- These recommendations are extrapolated to using TB-LAMP in children, based on the generalization of data from adults, while acknowledging the difficulties of collecting sputum specimens from children.
Test description
The amplification reaction requires four types of primers, which are complementary to six regions of the target gene. At about 65 °C, double-stranded DNA is in a condition of dynamic equilibrium. One of the LAMP primers can anneal to the complementary sequence of double-stranded target DNA, initiating DNA synthesis with the polymerase; strand displacement activity then displaces and releases a single-stranded DNA. Owing to the complementarity of the 5´-end of the forward inner primer (known as FIP) and the backward inner primer (BIP) in nearby regions of the target amplicon, loop structures are formed. This allows variously sized structures, consisting of alternately inverted repeats of the target sequence on the same strand, to be formed in rapid succession.
The addition of loop primers, which contain sequences complementary to the single-stranded loop region on the 5´-end of the hairpin structure, speeds the reaction by providing a greater number of starting points for DNA synthesis. Using loop primers, amplification by 109–1010 times can be achieved within 15–30 minutes. The version of TB-LAMP that was evaluated includes loop primers for a total of six primers binding to eight locations. This requirement for homogeneous sequences at multiple binding sites preserves the specificity of the assay, even in the absence of a probe.
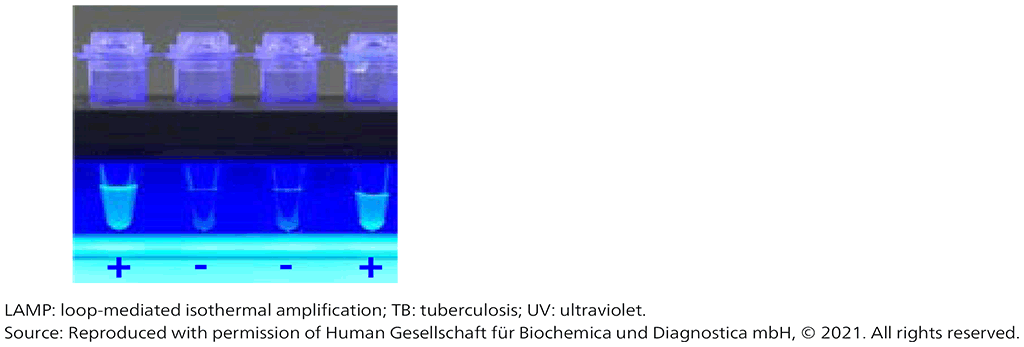
The LAMP method is relatively insensitive to the accumulation of DNA and DNA byproducts (pyrophosphate salts), so the reaction proceeds until large amounts of amplicon are generated. This feature makes it possible to visually detect successful amplification using double-stranded DNA-binding dyes, such as SYBR green, by detecting the turbidity caused by precipitating magnesium pyrophosphate or by using a non-inhibitory fluorescing reagent that is quenched in the presence of divalent cations. Fig. 2.2.1 shows calcein, unquenched by pyrophosphate consumption of divalent cations, fluorescing under UV light. The turbid, fluorescent product is easily seen with the naked eye.
Fig. 2.2.1. Visual display of TB-LAMP results under UV light
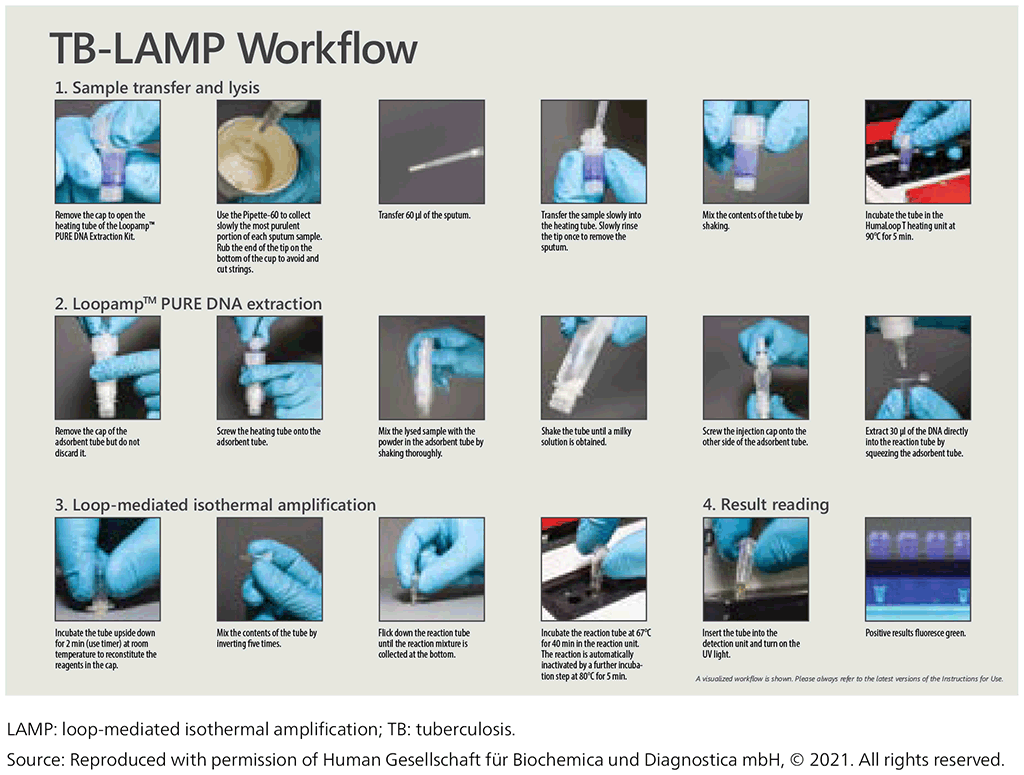
The test procedure has three main steps (Fig. 2.2.2):
- Sample preparation – bacteria are heat treated for inactivation and lysis. This step also includes the extraction of DNA.
- Amplification – the sample is placed in a heating block at 67 °C. At this temperature, the polymerase enzyme amplifies the target DNA.
- Visualization – the test-tube contains a double-stranded DNA-binding molecule that will fluoresce under UV light, meaning that detection can easily be performed with the naked eye.
Fig. 2.2.2. Description of the workflow for TB-LAMP
Justification and evidence
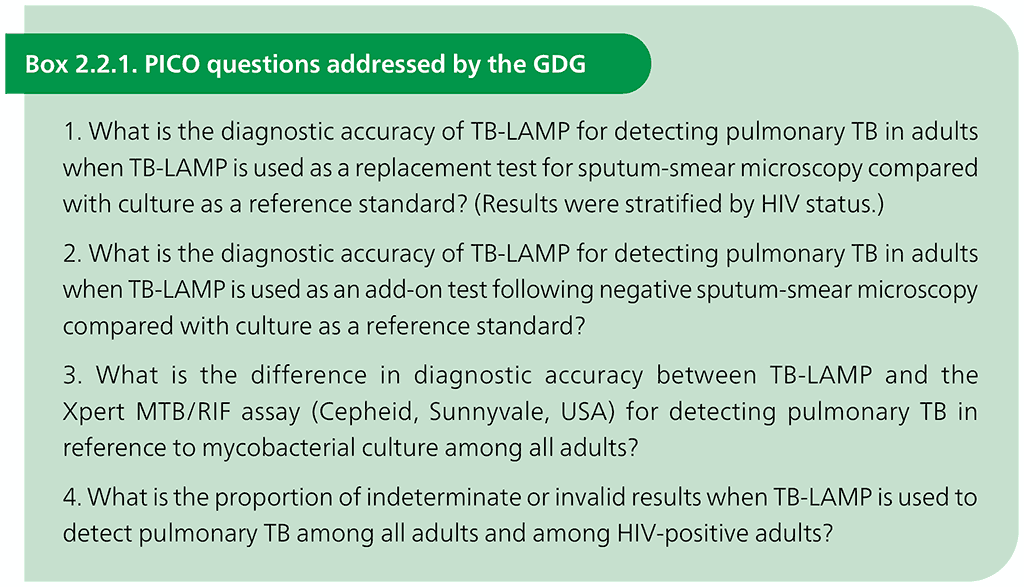
The evidence reviewed, and this policy guidance apply only to the use of the commercial TB-LAMP manual assay. In accordance with WHO’s standards for assessing evidence when formulating policy recommendations, the GRADE approach was used. GRADE provides a structured framework to determine the quality of the evidence and to provide information on the strength of the recommendations, using PICO questions agreed by the GDG. PICO refers to the following four elements that should be included in questions that govern a systematic search of the evidence: the population targeted by the action or intervention (in the case of systematic reviews of the accuracy of diagnostic tests, P is the population of interest), the intervention (I is the index test), the comparator (C is the comparator test or tests) and the outcomes (O is usually sensitivity and specificity). The PICO questions for the review are given in Box 2.2.1.
The review included all prospective studies that evaluated the use of TB-LAMP on sputum samples from adults with signs and symptoms consistent with pulmonary TB that were conducted in settings with an intermediate or high burden of TB. Twenty studies were identified, including all studies that were directly conducted by FIND or funded through FIND following a request for applications. Study participants who could not be classified as TB-positive or TB-negative based on the reference standard definitions described below were excluded.
The mycobacterial culture reference standards listed below were used to classify TB status. Eligible studies performed one or more sputum cultures on solid media (Löwenstein–Jensen) or on liquid media using the BACTEC™ mycobacterial growth indicator tube (MGIT; Becton Dickinson, Franklin Lakes, USA), or on both liquid and solid media. To account for the different number of cultures performed by studies and the different number of culture results available for participants, three hierarchical culture-based reference standards were used to assess diagnostic accuracy.
Standard 1 comprised:
- TB: at least one positive culture-confirmed to be MTBC by speciation testing.
- Not TB: no positive and at least two negative cultures performed on two different sputum samples.
Standard 2 comprised:
- TB: at least one positive culture-confirmed to be MTBC by speciation testing.
- Not TB: No positive and at least two negative cultures performed on at least one sputum sample.
Standard 3 comprised:
- TB: at least one positive culture-confirmed to be MTBC by speciation testing.
- Not TB: No positive and at least one negative culture.
Across the three standards, there is an expected trade-off between the yield of a confirmed TB diagnosis (highest with Standard 1 and lowest with Standard 3) and the number of studies or participants included in the analysis (lowest with Standard 1 and highest with Standard 3). Thus, using Standard 1, the potential for false negative index test results is highest and for false positive index test results is lowest. Also, using Standard 1, the number of studies and study participants included is expected to be lowest because it excludes studies that performed only one culture, and study participants for whom only one negative culture result was available due to culture contamination; in contrast, using Standard 3, the number of studies and study participants is highest.
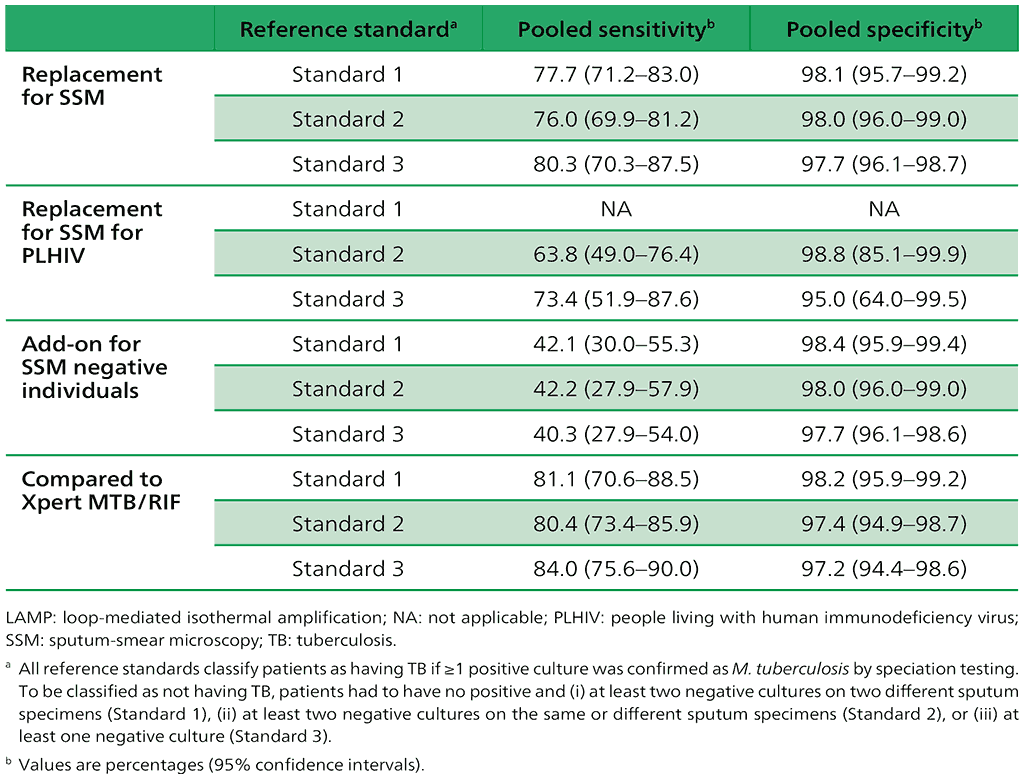
Of the 4760 adults eligible for inclusion in the analysis, 1810 participants (38%) across seven studies qualified for Standard 1 status, 3110 participants (65%) across 10 studies qualified for Standard 2 and 4596 participants (97%) across 13 qualified for Standard 3 (Table 2.2.1).
The performance of the test was calculated using the three different reference standards for the following scenarios:
- TB-LAMP as a replacement for sputum-smear microscopy;
- TB-LAMP as a replacement for sputum-smear microscopy among PLHIV;
- TB-LAMP as an add-on test for sputum-smear microscopy negative individuals; and
- TB-LAMP in head-to-head comparison with Xpert MTB/RIF.
Table 2.2.1. TB-LAMP as a replacement test for smear microscopy: estimates of pooled sensitivity and specificity
Details of studies included in the current analysis are given in Web Annex 1.4: TB-LAMP. Summary of the results and details of the evidence quality assessment are available in Web Annex 2.4: TB-LAMP.
Cost–effectiveness analysis
For the cost analysis, a bottom-up micro-costing analysis was conducted – the aim being to identify, measure and value all resources relevant to providing TB-LAMP and the Xpert MTB/RIF assay as routine diagnostic tests in peripheral laboratories in Malawi and Viet Nam. The two TB-LAMP strategies (used as a replacement test for sputum-smear microscopy and as an add-on test to sputum-smear microscopy for further testing in smear-negative patients) were compared with the base case algorithm, with sputum-smear microscopy followed by clinical diagnosis in those patients with a negative microscopy result.
The weighted average per-test cost of TB-LAMP was US$ 13.78–16.22, and for the Xpert MTB/RIF assay it was US$ 19.17–28.34 when these tests were used as routine diagnostic tests at all peripheral-level laboratories in both countries. The first-year expenditure required for implementation at peripheral laboratories with a medium workload (10–15 sputum-smear microscopy tests per day) in Viet Nam was US$ 26 917 for TB-LAMP and US$ 43 325 for the Xpert MTB/RIF assay. These costs were about US$ 3000 lower in Malawi, because of lower operating and staff costs. Likewise, TB-LAMP was a considerably cheaper test to implement, accounting for 9.33% of the reported TB control budget for 2014 in Malawi and 17.2% in Viet Nam; in comparison, implementing the Xpert MTB/RIF assay accounted for 18% of the reported TB control budget in Malawi and 37% in Viet Nam. In the cost–effectiveness analyses, both of the TB-LAMP scenarios improved case-detection rates, and both strategies were cost effective when compared with WHO’s willingness-to-pay threshold levels.
The cost–effectiveness analysis findings demonstrate that TB-LAMP is potentially a cost-effective alternative to the base case of sputum-smear microscopy plus clinical diagnosis in settings where the Xpert MTB/RIF assay cannot be implemented because of the infrastructure requirements, including a continuous power supply. However, given the inability of TB-LAMP to detect RR-TB, and its suboptimal sensitivity for detecting TB among PLHIV, national policy-makers must cautiously evaluate the operational feasibility and cost considerations before introducing this technology.
Implementation considerations
The systematic review supports the use of TB-LAMP as a replacement test for smear microscopy, for diagnosing pulmonary TB in countries with an intermediate or high burden of TB. However, the Xpert MTB/RIF assay should remain the preferred diagnostic test for anyone suspected of having TB, provided that there are sufficient resources and infrastructure to support its use, given the evidence, its ability to simultaneously identify rifampicin resistance and the fact that it is automated.
- Several operational issues accompany the implementation of TB-LAMP; for example, the need for electricity, adequate storage and waste disposal, stock monitoring and temperature control in storage settings where temperatures exceed the manufacturer’s recommendation (currently 30 °C for TB-LAMP).
- TB-LAMP is designed and has been evaluated to detect M. tuberculosis in sputum specimens. Its use with other samples (e.g. urine, serum, plasma, CSF or other body fluids) has not been adequately evaluated.
- Adoption of TB-LAMP does not eliminate the need for smear microscopy, which should be used for monitoring the treatment of patients with drug-susceptible TB. However, the demand for conventional sputum microscopy may decrease in settings where TB-LAMP fully or partially replaces conventional sputum microscopy.
- TB-LAMP should not replace the Xpert MTB/RIF assay because the latter simultaneously detects M. tuberculosis and rifampicin resistance, is automated and is relatively simple to perform.
- In settings where the Xpert MTB/RIF assay cannot be implemented (e.g. because of an inadequate electric supply, or excessive temperatures, humidity or dust), TB-LAMP may be a plausible alternative.
Research priorities
- Evaluation of diagnostic algorithms in different epidemiological and geographical settings and patient populations.
- Conducting of more rigorous studies with higher quality reference standards (including multiple specimen types and extrapulmonary specimens) to improve confidence in specificity estimates.
- Determination of training needs, and assessments of competency and quality.
- Gathering of more evidence on the impact on TB treatment initiation, morbidity and mortality.
- Performance of country-specific cost–effectiveness and cost–benefit analyses of targeted TB-LAMP use in different programmatic settings.
- Meeting the Standards for Reporting Diagnostic Accuracy Studies (STARD) for future studies (23).
 Feedback
Feedback