Book traversal links for Performance of SL-LPA on sputum specimens and culture isolates
In patients with MDR/RR-TB, a positive SL-LPA result for fluoroquinolone resistance (as a class) or SLID resistance (as a group) can be treated with confidence. The diagnostic accuracy of SL-LPA is similar when performed directly on sputum specimens or indirectly on cultured isolates of M. tuberculosis.
Given the confidence in a positive result and the ability of the test to provide rapid results, the GDG felt that SL-LPA may be considered for use as an initial test for resistance to the fluoroquinolones and when relevant SLIDs. However, when the test shows a negative result, phenotypic culture-based DST may be necessary, especially in settings with a high pretest probability for resistance to either fluoroquinolones or SLIDs (or both). The use of SL-LPA in routine care should improve the time to the diagnosis of fluoroquinolone and where relevant SLIDs, especially when used for the direct testing of sputum specimens of patients with confirmed MDR/RR-TB. Early detection of drug resistance should allow for the earlier initiation of appropriate patient therapy and improved patient health outcomes. Overall, the test performs well in the direct testing of sputum specimens from patients with confirmed MDR/RR-TB, although the indeterminate rate is higher when testing smear-negative sputum specimens compared with smear-positive sputum specimens.
When the MTBDRsl assay is used in the direct testing of smear-negative sputum specimens from a population of patients with confirmed DR-TB, up to 44% of the results may be indeterminate (less with version 2.0, although very limited data) and hence require repeat or additional testing.
However, if the same test were to be applied to the testing of smear-negative sputum specimens from patients without confirmed TB or DR-TB (i.e. patients suspected of having DR-TB), the indeterminate rate for the test would be significantly higher. Given the test’s sensitivity and specificity when an SL-LPA is done directly on sputum, the GDG felt that SL-LPAs can be used for the testing of all sputum specimens from patients with confirmed MDR/RR-TB, irrespective of whether the microscopy result is positive or negative.
Table 2.3.4. Accuracy of GenoType MTBDRsl (version 1.0) for fluoroquinolone and SLID resistance and XDR-TB, indirect and direct testing (smear-positive specimens), culture-based DST reference standard
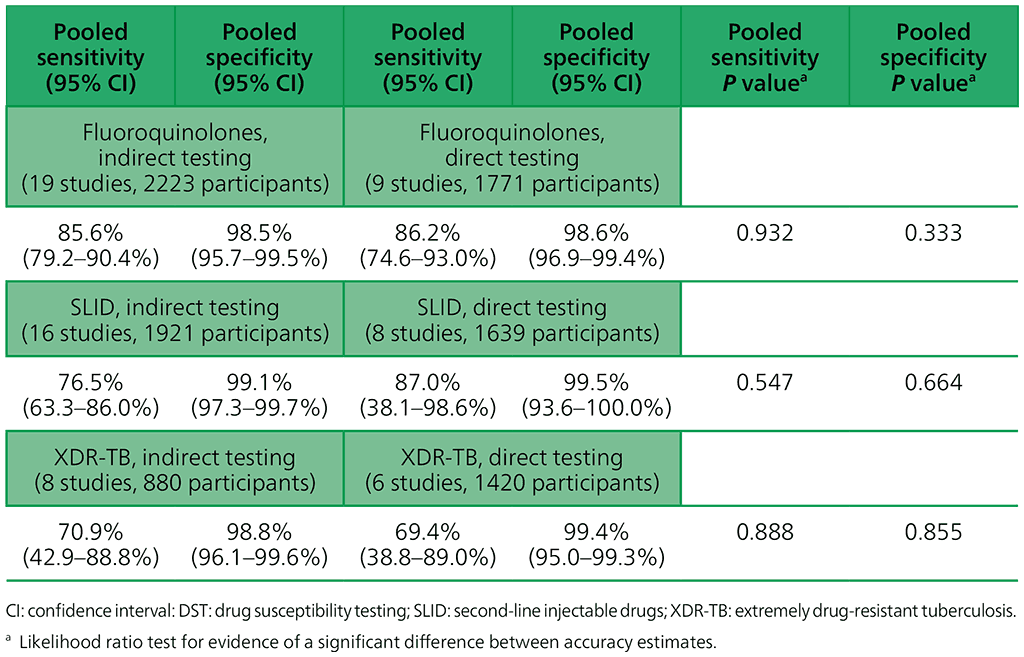
For the reasons mentioned above (inadequate data owing to too few studies on version 2.0), results are not presented here for version 2.0. For MTBDRsl version 2.0, the data were either too sparse or too heterogeneous to combine in a meta-analysis or to compare indirect and direct testing.
Three studies evaluated the MTBDRsl version 2.0 in 562 individuals, including 111 confirmed cases of TB with fluoroquinolone resistance by indirect testing on a culture of M. tuberculosis compared with a phenotypic culture-based DST reference standard. Estimates of sensitivity ranged from 84% to 100% and specificity from 99% to 100%.
See Web Annex 4.16: Drug concentrations used in culture-based DST SL-LPA for details of the drug concentrations used in culture-based DST to evaluate the performance of SL-LPAs in each included study.
Implementation considerations
The SL-LPA should only be used to test specimens from patients with confirmed MDR/RR-TB. Adoption of SL-LPAs does not eliminate the need for conventional culture and DST capability. Despite good specificity of SL-LPAs for the detection of resistance to fluoroquinolones and the SLIDs, culture and phenotypic DST is required to completely exclude resistance to these drug classes as well as to other second-line drugs. The following implementation considerations apply:
- SL-LPAs cannot determine resistance to individual drugs in the class of fluoroquinolones. Resistance-conferring mutations detected by SL-LPAs are highly correlated with phenotypic resistance to ofloxacin and levofloxacin.
- Mutations in some regions (e.g. the eis promoter region) may be responsible for causing resistance to one drug in a class more than other drugs within that class. For example, the eis C14T mutation is associated with kanamycin resistance in strains from Eastern Europe.
- SL-LPAs should be used in the direct testing of sputum specimens, irrespective of whether samples are smear negative or smear positive.
- SL-LPAs are designed to detect TB and resistance to fluroquinolones and SLIDs from sputum samples. Other respiratory samples (e.g. bronchoalveolar lavage and gastric aspirates) or extrapulmonary samples (tissue samples, CSF or other body fluids) have not been adequately evaluated.
- Culture and phenotypic DST plays a critical role in the monitoring of a patient’s response to treatment, and in detecting additional resistance to second-line drugs during treatment.
- SL-LPAs are suitable for use at the central or national reference laboratory level; they can also be used at the regional level if the appropriate infrastructure can be ensured (three separate rooms are required).
- All patients identified by SL-LPAs should have access to appropriate treatment and ancillary medications.
Research priorities
- Development of improved understanding of the correlation between the detection of resistance-conferring mutations with phenotypic DST results and with patient outcomes.
- Development of improved knowledge of the presence of specific mutations detected with SL-LPA correlated with minimum inhibitory concentrations for individual drugs within the classes of fluoroquinolones and SLIDs.
- Determination of the limit of detection of SL-LPA for the detection of heteroresistance.
- Gathering of more evidence on the impact of MTBDRsl on appropriate MDR-TB treatment initiation and mortality.
- Strongly encourage that future studies follow the recommendations in the STARD (42) statement to improve the quality of reporting.
- Performance of country-specific cost–effectiveness and cost–benefit analyses of the use of SL-LPA in different programmatic settings.
 Feedback
Feedback