Book traversal links for 4.2.5 Modification of treatment
The only experience using these new regimens stems from two clinical trials; it is therefore suggested that the programmatic implementation be aligned with this experience. Safe management of adverse events may warrant dose reduction or discontinuation of the component drugs. However, the BPaLM/ BPaL regimen has been studied as a standardized course of treatment. Modification of the regimen through early discontinuation or replacement of any of the component drugs may result in poor treatment outcomes. Although dose modification of bedaquiline and pretomanid is not recommended, dose modification of linezolid is acceptable after the first 9 weeks of treatment in cases of adverse events. Although dose modification of linezolid should be avoided in the first 9 weeks of therapy, this principle should not override the need to avoid permanent disabilities. In some circumstances, the linezolid may need to be permanently stopped and a decision made on whether to continue the other drugs to complete treatment or start a new treatment. After 9 weeks of consecutive administration of linezolid, the dose of linezolid can be reduced to 300 mg if necessary (see examples in Box 4.1).
Modifications of linezolid dose were made in the ZeNix and TB-PRACTECAL trials when linezolidassociated toxicity was suspected. Although no analysis was undertaken to determine whether individuals with dose reductions had poorer treatment outcomes than those who continued for the complete duration, overall treatment success was very high in all investigational arms.
Pharmacokinetic studies have suggested that dose optimization of linezolid may differ among patients (24). Therapeutic drug monitoring (TDM) is a novel approach that can be used, where available, to optimize linezolid dose and minimize adverse events, without compromising effectiveness (25).
Further research is encouraged and is ongoing, to help ascertain how to optimize TDM in the treatment of individuals with MDR/RR-TB who are prescribed linezolid.
A dosing strategy study evaluating Nix-TB study data suggested that monitoring neuropathy symptoms and haemoglobin level may help guide linezolid dosing to avoid toxicities. A decrease in haemoglobin level of 10% or more after 4 weeks of treatment may help to identify those at high risk for severe anaemia (23). However, further research on dosing strategies is needed to optimize when and how to reduce the dose of linezolid.
Regarding the cessation of any component drug of the BPaLM/BPaL regimen because of severe toxicity, the following factors should be taken into account:
- if either bedaquiline or pretomanid needs to be permanently discontinued, the entire BPaLM/BPaL regimen should also be discontinued;
- if linezolid is permanently discontinued during the initial 9 consecutive weeks of treatment, the entire regimen should be discontinued;
- if linezolid is withheld in the later weeks of the regimen, with the total remaining duration of the regimen not exceeding 8 weeks, the regimen can be considered to be completed with the remaining component drugs; and
- if moxifloxacin alone is discontinued, the regimen can be continued as the BPaL regimen.
If linezolid (or any of the drugs) is being intermittently stopped and started on the BPaLM/BPaL regimen, there may be concern for development of resistance to the other component medicines in the regimen. This may be more of a concern for patients receiving BPaL because during single drug disruption the regimen will consist of only two effective drugs. Acquired resistance to the two remaining agents in pre-XDR-TB cases can be catastrophic to both the patient and the society.
Although there is no specific number of missed regimen dosages to automatically indicate when the BPaLM/BPaL regimen should be declared a failure and an individualized longer regimen should be used instead, this general guidance to consider clinical review and switch to an individualized longer regimen should be considered when:
- more than 2 weeks of consecutive treatment interruption of all medicines in the regimen occurs; or
- more than 4 weeks cumulative of nonconsecutive treatment interruption of all medicines in the regimen occurs.
Extension of BPaL to 9 months should be done with caution in patients with a high number of missed linezolid dosages – switching to an individualized longer regimen may be considered instead of a BPaL extension.
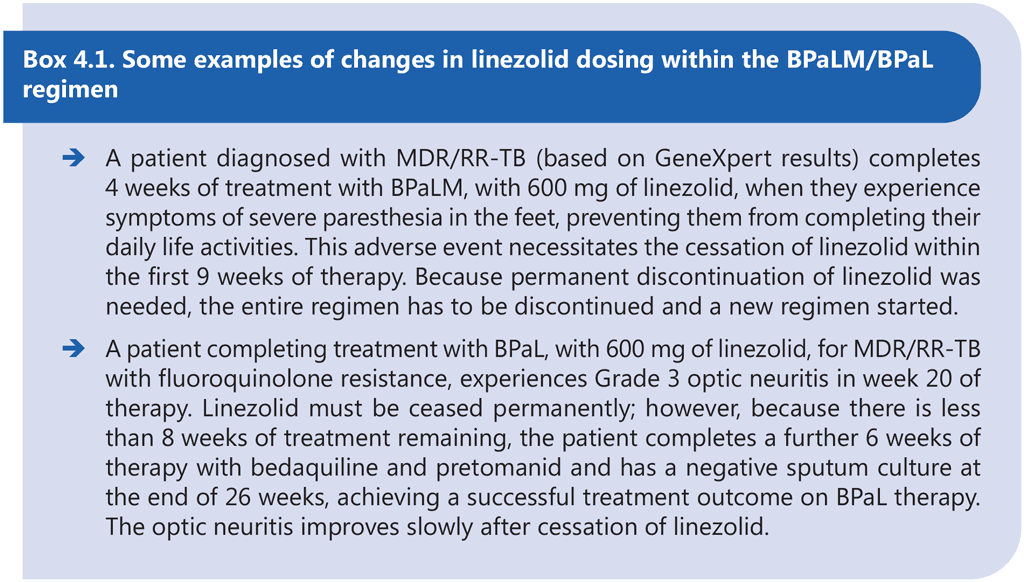
 Feedback
Feedback