Book traversal links for 1.1.1 Target Audience and Scope:
The main target audience for these policy guidelines consists of policy makers in member states as well as all employers of health workers. The recommended policy guidelines are expected to be useful for health and labour departments, regional policy-makers, health facility managers, and all front-line health workers - including informal health givers. It is expected that these guidelines will also be useful to representatives of health workers, including unions and health professional associations, as well as occupational health and infection control practitioners.
The guidelines were scoped to provide guidance for the target audience on how to implement interventions to promote policies and programmes where change is desired. It is important to stress that. these guidelines will not prescribe new clinical practices, but rather will refer to recently published guidelines on such clinical topics as post exposure prophylaxis (PPE) to prevent HIV infection (ILO/WHO 2008), health services and HIV (ILO/WHO2005), TB infection control in healthcare facilities, congregate settings, and households (WHO 2009), and others (see Annex 1) for listing of some of the relevant WHO and other international Guidelines referenced). These guidelines were developed to address the identified need to integrate existing guidelines relevant to health workers who are living with, or have been affected by HIV and/or TB, or who are at increased risk from HIV and TB as a result of occupational exposures, into one comprehensive source that taken together will improve access of health workers to prevention, treatment, care and support services for HIV and TB.
As such, the guidelines are "implementation" guidelines -- developed to provide the target audience with policy and programme guidance in how to effectively synthesize all the relevant existing clinical practice and related guidelines in the area, to lead to the desired outcome. Attention to the refinement of implementation strategies in this area is analogous to the increased research focus on "implementation research". As there is a growing body of scientific study of methods to promote the uptake of clinical research findings into routine practice (Eccles 2009), so too has there been a growing body of knowledge on how to implement the complex multi-level interventions (Medical Research Council 2006), necessary for the uptake of health service and population health measures to achieve the outcomes of interest.
The process used to develop these guidelines reflects the state-of-the-art thinking on how to synthesize information for decision-making regarding complex multi-component public health interventions, (Shepperd 2009, Eccles 2009, Cochrane Public Health Review Group http://www.ph.cochrane.org/en/index.html, Cochrane Health Equity Field- http://equity.cochrane.org/en/index.html), as discussed below. It thus represents an attempt to synthesize a complex body of knowledge currently scattered across numerous different documents.
Following the process articulated in the WHO Handbook on Guideline Development (2010), a multidisciplinary group was formed consisting of the key stakeholder departments and organizations that have expertise and interest in achieving the desired outcome. This Guideline Group (GG) led the guideline development process, as outlined in the next section.
Methodology
The systematic review of evidence conducted for developing these guidelines had multiple components, as shown in Figure 1.
Figure 1: Components of the Systematic Evidence Review
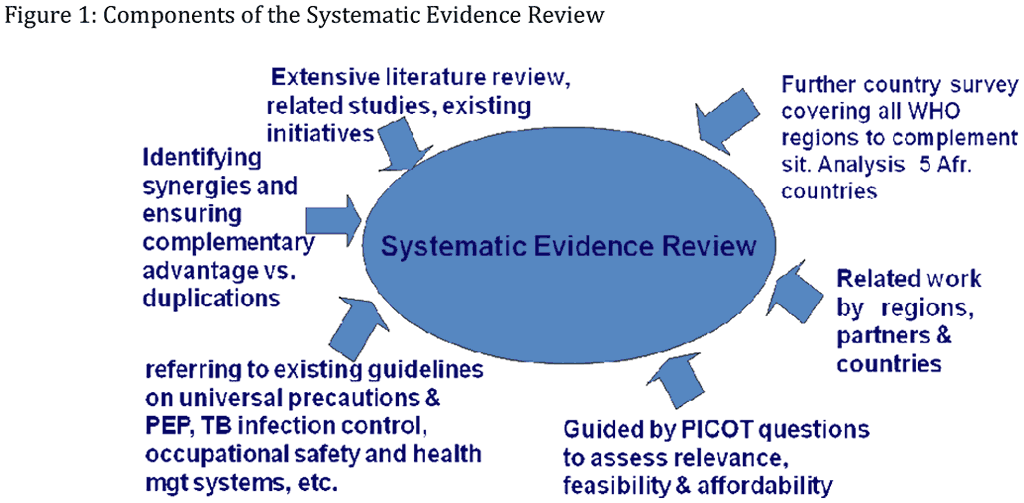
As noted by Shepperd et al. (2009) complex health interventions are difficult to evaluate using the traditional Cochrane approach, particularly when the interventions include elements of a more conceptual nature, such as "trust", or in this case "reducing stigma" and preventing "discrimination". The authors note that policy documents can be particularly informative, consistent with the approach of the Guideline Group's commissioning of a policy review across all regions of the world, as described below. One component follows the Cochrane review style of systematic reviews. As the GG recognized the limitations of the traditional more clinically-oriented Cochrane approach for this complex task of largely synthesizing existing guidelines, putting these into context of current needs and realities, the systematic evidence review for development of this guidelines consisted of multiple components, as outlined below.
 Feedback
Feedback