Book traversal links for 5.2.7.3. Dosing table for the short intensive TB meningitis regimen
The recommended dosages by weight band for the 6-month intensive regimen (6HRZEto) to treat bacteriologically confirmed or clinically diagnosed TBM (without suspicion or evidence of MDR/RR-TB) in children and adolescents weighing less than 35 kg are shown in Table 5.6. These dosages were developed to limit formulation manipulation (splitting tablets), top-up with standalone medicines, number of weight bands and pill burden.
For children weighing less than 25 kg, it is preferable to use child-friendly dispersible tablet formulations, including the HR FDC. For children weighing 3–<5 kg, a joint age- and weight-based approach was adopted, accounting for maturation factors. The dosing of pyrazinamide and isoniazid and rifampicin for children weighing 3–<5 kg depends on whether the child is aged under or over 3 months.
For children weighing 25–<35 kg, either dispersible tablets or adult formulations of the corresponding medicines (HR 75/150 mg, Z 400 mg or 500 mg, Eto 250 mg) can be used. Using adult formulations in older children reduces the number of pills. For severely ill children, such as those with a reduced level of consciousness, child-friendly dispersible formulations can be administered via a nasogastric tube. Table 5.6 also provides dosing options for both Z 400 mg and Z 500 mg, acknowledging that NTPs may procure either formulation.
Ethionamide can cause hepatotoxicity, gastrointestinal irritability and hypothyroidism. Gastrointestinal irritability can mostly be overcome by dosing ethionamide in the evening, separately from other TB medicines.
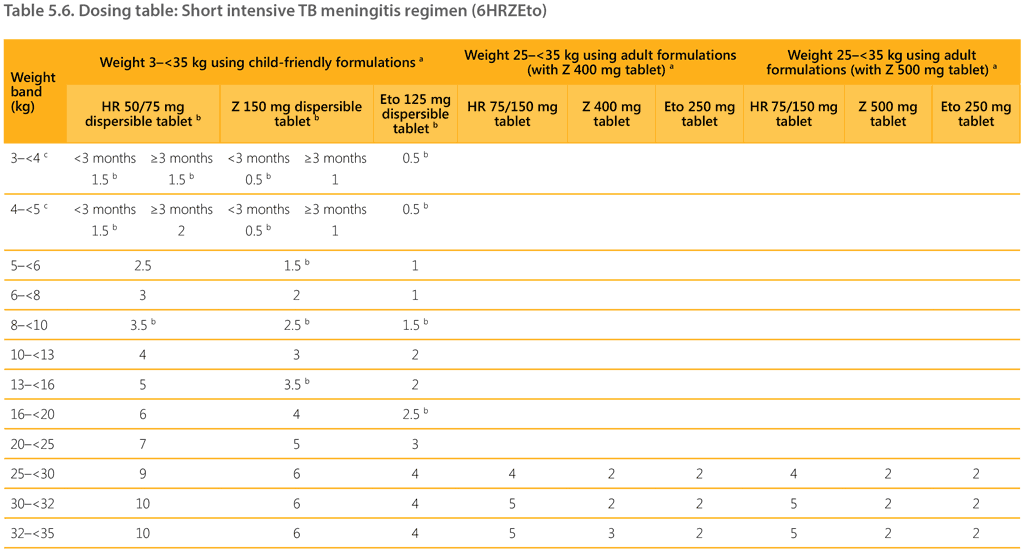
a For children weighing 25–<35 kg, adult formulations can be used to reduce the pill burden.
b If the formulation has a scoring line, tablets can be split and administered whole or dispersed in water. If the formulation does not have a scoring line, tablets should be dispersed in a specific amount of water and the exact dose administered using an aliquot with a syringe. To give 0.5 tablet, dissolve 1 tablet in 10 mL water and administer 5 mL.
c For children weighing 3–<5 kg, a joint age- and weight-based approach is used. The dosing of RH and Z for children weighing 3–<5 kg depends on whether the child is aged under or over 3 months. For example,
an infant weighing 4.5 kg would receive 1.5 tablets of HR 50/75 mg and 0.5 tablets of Z 150 mg if aged under 3 months, but 2 tablets of HR and 1 tablet of Z if aged 3 months or over.
 Feedback
Feedback