Book traversal links for Annex 6. Dosing of medicines used in second-line multidrug-resistant TB regimens by weight band (below 46 kg)
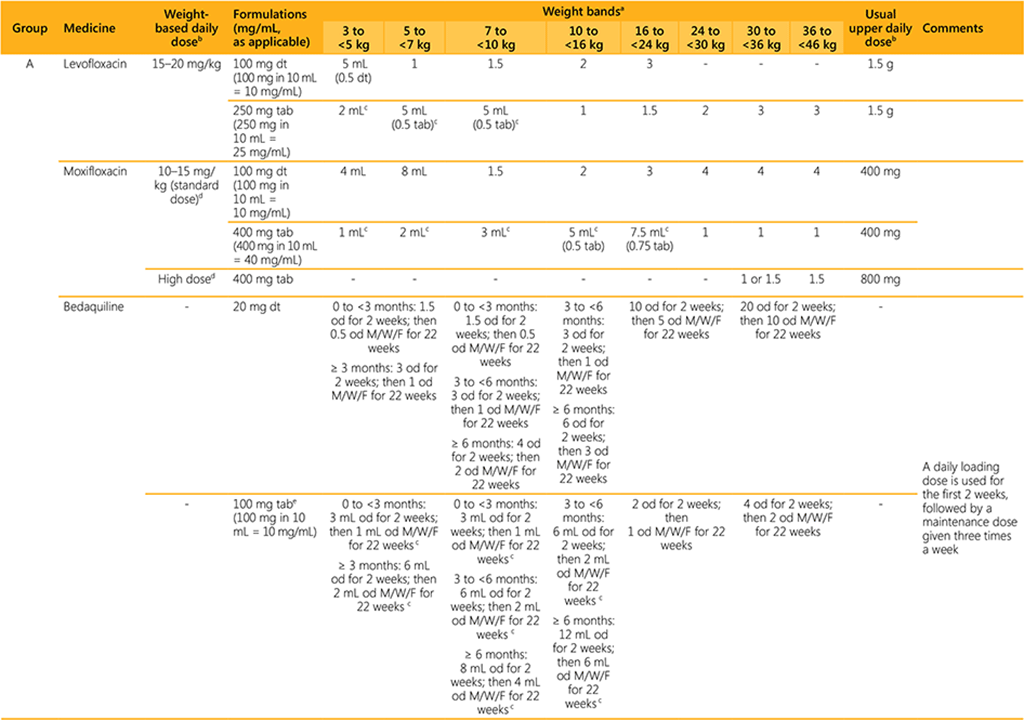
- Dosing guidance is based on currently available data and may be revised once additional data are available.
- For patients weighing ≥46 kg, please refer to Table A in Annex 1 of the WHO operational handbook on tuberculosis. Module 4: treatment – drug-resistant tuberculosis: Dosing of medicines used in second-line multidrug-resistant TB regimens by weight band (patients 15 years or older).
- For dosing of premature and low birth weight infants weighing <3 kg, advice from a paediatric DR-TB expert should be sought.
- For dosing of infants weighing 3 to <5 kg, a paediatric DR-TB expert should be consulted whenever possible.
- The use of child-friendly, dispersible tablets in infants and young children is preferred over manipulating adult tablets or administering/manipulating capsules. Where applicable, the dosing provided is based on dissolving the dispersible formulation in 10 mL of water and administering the number of mL (aliquots). The dissolved solution should be used immediately and the remainder of the 10 mL should be discarded.
- For some weight bands, dosing is indicated with both child-friendly, dispersible formulations and adult formulations. If adult formulations are used, the table provides the dose using aliquots in mL and tablet fractions where applicable (if the fraction is 0.5 or more). Aliquots refer to the volume to administer after crushing and dissolving the tablet in 10 mL of water.
AEs: adverse events; bd: two times a day; cap: capsule; dt: dispersible tablet; g: gram; kg: kilogram; mL: milliliter; mg: milligram; M/W/F: Monday, Wednesday, Friday; od: once daily; soln: solution; susp: suspension; tab: tablet.
a Dosages were established by the guideline development groups for the WHO guidelines on drug-resistant tuberculosis treatment (2018 and 2020 updates), the WHO Global Task Force on the Pharmacokinetics
and Pharmacodynamics (PK/PD) of TB medicines and the expert group convened after the guideline development group on child and adolescent TB in 2021 and 2022. They are based on the most recent reviews
and best practices in the treatment of (paediatric) MDR/RR-TB. For certain medicines the dosages were informed by pharmacokinetic modelling results based on the principle of allometric scaling and maturation
(Denti P, Wasmann RE, Francis J, et al. One dose does not fit all: revising the WHO paediatric dosing tool to include the non-linear effect of body size and maturation. Lancet Child Adolesc Health. 2022;6(1):9–
10). Due to the pharmacokinetic properties of certain medicines the doses proposed may exceed the mg/kg/day ranges shown here in order to achieve blood concentrations similar to target levels in an average
adult patient. The guidance for the 3-<5kg weight band and for bedaquiline and delamanid is based on currently available data and may be revised when new data becomes available.
b Clinicians may decide to exceed these values in particular cases to improve therapeutic effect. In infants weighing less than 10 kg, consultation with an expert in paediatric DR-TB is advised, in view of limited data
on maturation.
c Dissolving of crushed adult tablets or capsule content in 10 mL of water is required for administering this dose. The number of mL in the table reflects the dose to provide. This avoids fractioning solid formulations,
although bioavailability of the dissolved, crushed adult tablets is uncertain (use of dispersible tablets is preferred if available).
d The higher dose may be used except when: there is risk of toxicity; levels are expected to be lowered because of pharmacokinetic interactions, malabsorption or other reasons; or the strain has low-level
drug resistance.
e Bedaquiline adult tablets (100 mg) crushed and suspended in water have been shown to be bioequivalent to tablets swallowed whole. Vigorous stirring/shaking is needed prior to administering the 100 mg tablet crushed and suspended in water.
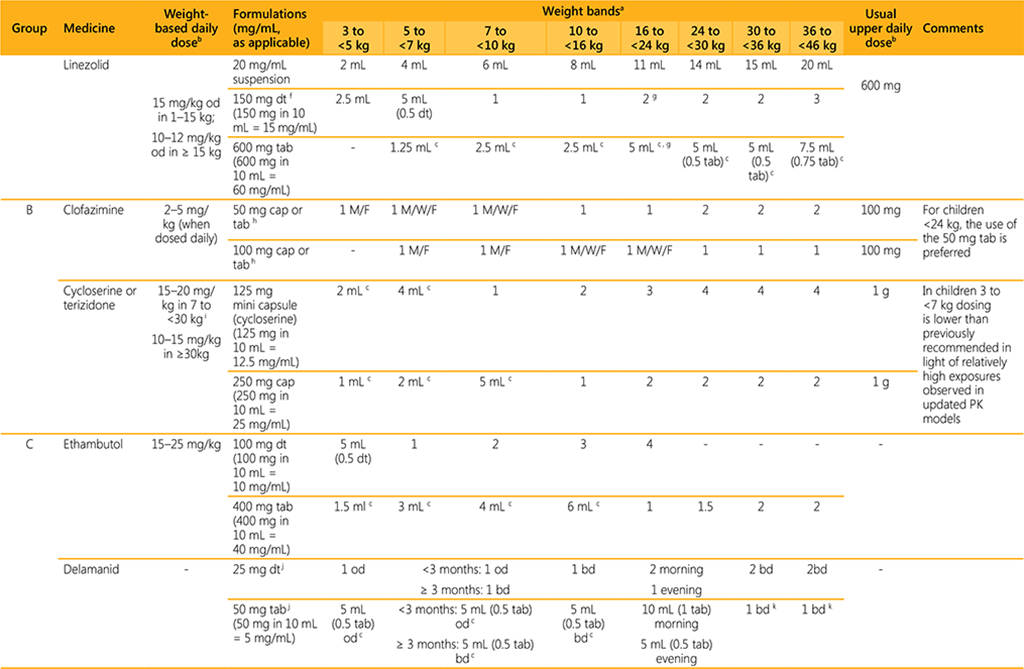
f The 150 mg dispersible tablet for linezolid is expected to become available in 2022.
g When using the 600 mg tab and the 150 mg dt to dose children weighing 16 to <24 kg, the dose in mg/kg will exceed 10–12 mg/kg and clinicians may opt to administer 1.5 dt or 4 mL of the 600 mg tab dispersed
in 10 mL of water.
h Clofazimine tablets are technically not dispersible but they do slowly (this takes approximately 5 minutes) dissolve in water (5 mL and 10 mL for the 50 mg and 100 mg tablets, respectively). The suspension should
be stirred prior to administration. The 100 mg soft gel capsule is difficult to swallow for young children and therefore countries are strongly encouraged to make the 50 mg tablet formulation available.
i In children weighing 3 to <7 kg doses are lower than previously recommended. This is because of relatively high exposures associated with risk of neuropsychiatric AEs, which is especially concerning when
co-administering cycloserine with delamanid
j Delamanid adult tablets (50 mg) crushed and suspended in water have been shown to be bioequivalent to tablets swallowed whole.
k The dose for delamanid in children and young adolescents weighing 30 to < 46 kg differs from the dose provided for older adolescents and adults in the same weight band in Table A (Module 4: Operational
Handbook DR-TB).
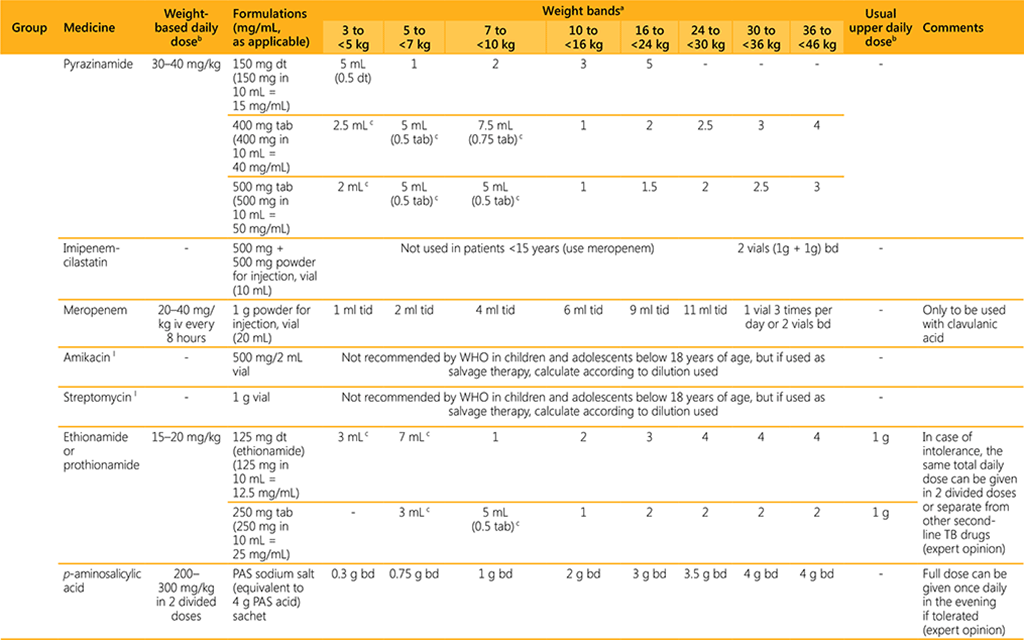
l Amikacin and streptomycin may be used in adults aged 18 years or more, in situations where an effective regimen cannot otherwise be designed using oral agents, when susceptibility is demonstrated, and
when adequate measures are in place to monitor for adverse events. Given the profound impact that hearing loss can have on the acquisition of language and the ability to learn at school, the use of injectable
agents in children should be exceptional and limited to salvage therapy, and the treatment needs to be provided under strict monitoring to ensure early detection of ototoxicity. If used, the weight-based daily
dose for amikacin is 15–20 mg/kg and for streptomycin is 20–40 mg/kg for children over 2 years. To determine the dosing for infants and children < 2 years, a paediatric DR-TB expert should be consulted and
a lower mg/kg dose used to compensate for immature clearance. Co-administration with lidocaine is advised to reduce pain at injection site (Garcia-Prats AJ, Rose PC, Draper HR et al. Effect of Coadministration
of Lidocaine on the Pain and Pharmacokinetics of Intramuscular Amikacin in Children with Multidrug-Resistant Tuberculosis: A Randomized Crossover Trial. Pediatr Infect Dis J. 2018 Dec;37(12):1199–1203).
m These medicines are only recommended as a companion agent (amoxicillin/clavulanic acid) or are not included in groups A, B and C, because of a lack of data from the latest analysis on longer MDR-TB regimens
in adults (isoniazid).
n In infants, pyridoxine may be given as part of a multi-vitamin syrup.
o Only to be used with the carbapenems, and only available in combination with amoxicillin as co-amoxiclav. For example, for the 24 to <30 kg weight band, amoxicillin/clavulanate, 500/125 mg bd is given.
 Feedback
Feedback