Book traversal links for 4.2 Algorithm 2 – LF-LAM testing to aid in the diagnosis of TB among PLHIV
Algorithm 2 is the preferred algorithm for testing to support the diagnosis of TB in PLHIV. It is appropriate for use in settings with a high burden of HIV and for use with individual PLHIV who meet the testing criteria, regardless of the overall HIV burden. The algorithm emphasizes the use of LF-LAM to quickly identify people needing TB treatment; it also emphasizes that all individuals with signs and symptoms of TB should receive a rapid mWRD (Algorithm 1). LF-LAM results (test time <15 minutes) are likely to be available before mWRD results, and treatment decisions should be based on the LF-LAM result while awaiting the results of other diagnostic tests.
The currently available LF-LAMs have sufficient sensitivity and specificity to aid in the diagnosis of TB among individuals coinfected with HIV, but have suboptimal sensitivity and specificity in those who are HIV-negative. Hence, this algorithm emphasizes the use of the LF-LAM test as a diagnostic test in all PLHIV with signs and symptoms of TB, as well as in other specific scenarios (described below) for the diagnosis of TB among PLHIV (52). The ease of use of the LF-LAM test makes it suitable for implementation outside of the laboratory – for example, in clinics (especially in those that see critically ill PLHIV) – for rapid diagnosis of TB and treatment initiation in urgent cases of suspected TB among PLHIV. Algorithm 2a is used for PLHIV being evaluated for TB (pulmonary or extrapulmonary) in an inpatient setting. The updated WHO screening guidelines now recommend adult and adolescent inpatients with HIV in medical wards where the TB prevalence is more than 10% should be tested systematically for TB disease with an mWRD, as described in Algorithm 1 (9). This is in addition to the recommendations on the use of LF-LAM among inpatient PLHIV (49). Algorithm 2b is used for PLHIV being evaluated for TB (pulmonary or extrapulmonary) in an outpatient setting or clinic.
These algorithms are appropriate for both low and high MDR-TB or Hr-TB burden settings. The choice of which molecular test to use may be different in a low or high MDR-TB or Hr-TB burden setting, as discussed under Algorithm 1. For example, in a setting with a high burden of MDR-TB, it would be preferable to use an mWRD that detects MTBC and RIF resistance simultaneously (e.g. Xpert MTB/RIF), rather than an mWRD that uses a two-step process (e.g. Truenat MTB) to detect RIF resistance.
Fig. 4.3. Algorithm 2a: LF-LAM to aid in the diagnosis of TB among PLHIVᵃ in inpatient settings
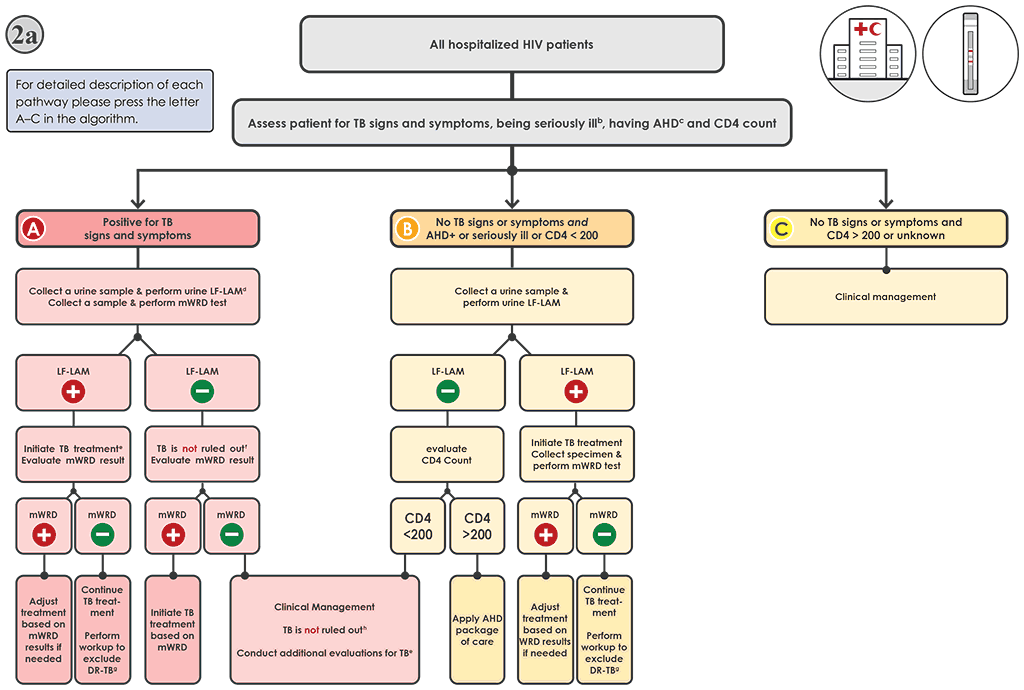
AHD: advanced HIV disease; AIDS: acquired immunodeficiency syndrome; DNA: deoxyribonucleic acid; DR-TB: drug-resistant tuberculosis; DST: drug susceptibility testing; FQ: fluoroquinolone; HIV: human immunodeficiency virus; LF-LAM: lateral flow lipoarabinomannan assay; LPA: line-probe assay; MC-aNAAT: moderate complexity automated nucleic acid amplification test; MDR-TB: multidrug-resistant tuberculosis; mWRD: molecular WHO-recommended rapid diagnostic test; PLHIV: people living with HIV/AIDS; TB: tuberculosis; WHO: World Health Organization; WRD: WHO-recommended rapid diagnostic test.
a PLHIV include people who are HIV-positive or whose HIV status is unknown but who present with strong clinical evidence of HIV infection, reside in settings where there is a high prevalence of HIV or are members of a group at risk for HIV. For all people with unknown HIV status, HIV testing should be performed in accordance with national guidelines. PLHIV with TB may also present with signs and symptoms of extrapulmonary TB, including lymphadenopathy, meningitis or other atypical presentations that warrant evaluation.
b “Seriously ill” is defined based on four danger signs: respiratory rate >30 per minute, temperature >39 °C, heart rate >120 beats per minute and unable to walk unaided.
c For adults, adolescents and children aged >5 years, AHD is defined as CD4 cell count <200 cells/mL3, or WHO stage 3 or 4 event at presentation for care. All children aged <5 years are considered as having AHD.
d The LF-LAM test and mWRD should be done in parallel. The results of the LF-LAM (test time <15 minutes) are likely to be available before the mWRD results; hence, treatment decisions should be based on the LF-LAM result while awaiting the results of other diagnostic tests.
e People should be initiated on a first-line regimen according to national guidelines, unless they are at very high risk of having MDR-TB (In whichh case, they should be initiated on an MDR-TB regimen).
f Negative LF-LAM results do not rule out TB in people with symptoms. The mWRD result should be evaluated when it becomes available for treatment decisions. See Algorithm 1 for interpretation of mWRD results.
g Phenotypic (culture and DST) and molecular (e.g. mWRDs, LPAs and DNA sequencing) methods are available to evaluate drug resistance. Rapid molecular methods (e.g. Xpert MTB or Truenat MTBRIF Dx or MC-aNAAT tests) are preferred.
h Negative mWRD and LF-LAM results do not rule out TB in people with symptoms; In such cases, additional clinical evaluations for TB should be carried out. Further investigations for TB may include chest X-ray, additional clinical assessments, clinical response following treatment with broad-spectrum antimicrobial agents, and additional mWRD testing or culture. Initiation of treatment for bacterial infections using antibiotics with broad-spectrum antibacterial activity (FQs should not be used) and for Pneumocystis pneumonia should be considered. The clinical response should be evaluated after 3–5 days of treatment.
Fig. 4.4. Algorithm 2b: LF-LAM testing to aid in the diagnosis of TB among PLHIVᵃ in clinic and outpatient settings
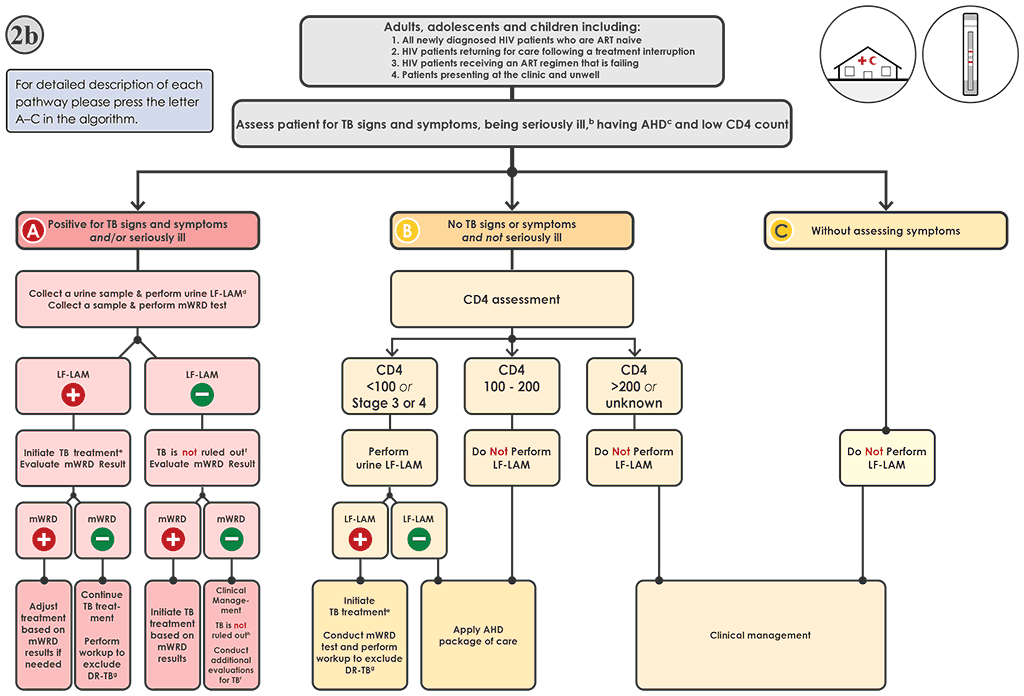
AHD: advanced HIV disease; ART: antiretroviral therapy; DNA: deoxyribonucleic acid; DR-TB: drug-resistant tuberculosis; DST: drug susceptibility testing; FQ: fluoroquinolone; HIV: human immunodeficiency virus; LF-LAM: lateral flow lipoarabinomannan assay; LPA: line-probe assay; MC-aNAAT: moderate complexity automated nucleic acid amplification test; MDR-TB: multidrug-resistant tuberculosis; mWRD: molecular WHO-recommended rapid diagnostic test; PLHIV: people living with HIV/AIDS; RIF: rifampicin; TB: tuberculosis; WHO: World Health Organization; WRD: WHO-recommended rapid diagnostic test.
a PLHIV include people who are HIV-positive or whose HIV status is unknown but who present with strong clinical evidence of HIV infection, reside in settings where there is a high prevalence of HIV or are members of a group at risk for HIV. For all people with unknown HIV status, HIV testing should be performed in accordance with national guidelines. PLHIV with TB may also present with signs and symptoms of extrapulmonary TB, including lymphadenopathy, meningitis or other atypical presentations warranting evaluation.
b “Seriously ill” is defined based on four danger signs: respiratory rate >30 per minute, temperature >39 °C, heart rate >120 beats per minute and unable to walk unaided.
c For adults, adolescents and children aged >5 years, AHD is defined as CD4 cell count <200 cells/mL3 or WHO stage 3 or 4 event at presentation for care. All children aged <5 years are considered as having AHD.
d The LF-LAM test and mWRD should be done in parallel. The results of the LF-LAM (test time <15 minutes) are likely to be available before mWRD results, and treatment decisions should be based on the LF-LAM result while awaiting the results of other diagnostic tests.
e People should be initiated on a first-line regimen according to national guidelines, unless the person is at very high risk of having MDR-TB (In which case, the person should be initiated on an MDR-TB regimen). Treatment regimens should be modified as needed based on the results of the mWRD testing.
f LF-LAM negative results do not rule out TB in people with symptoms. The result of the mWRD should be evaluated when it becomes available (see Algorithm 1 for interpretation of mWRD results).
g Phenotypic (culture and DST) and molecular (e.g. LPAs, DNA sequencing and high-throughput assays) methods are available to evaluate drug resistance. Rapid molecular methods (e.g. Xpert MTB or Truenat MTB-Truenat MTB Plus or MC-aNAATs) are preferred.
h The mWRD negative and LF-LAM negative results do not rule out TB in people with symptoms. Additional clinical evaluations for TB should be conducted. Further investigations for TB may include chest X-ray, additional clinical assessments, and additional mWRD testing or culture. Initiation of treatment for bacterial infections using antibiotics with broad-spectrum antibacterial activity (not FQs) and those for Pneumocystis pneumonia should be considered. The clinical response should be evaluated after 3–5 days of treatment.
 Feedback
Feedback