Book traversal links for Annex 1. Supplementary table
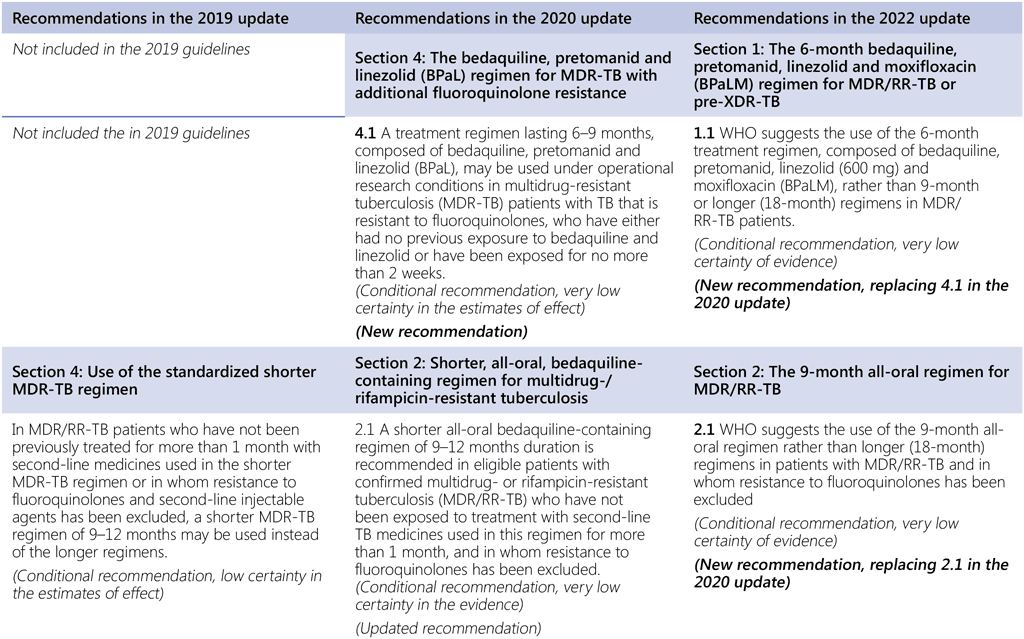
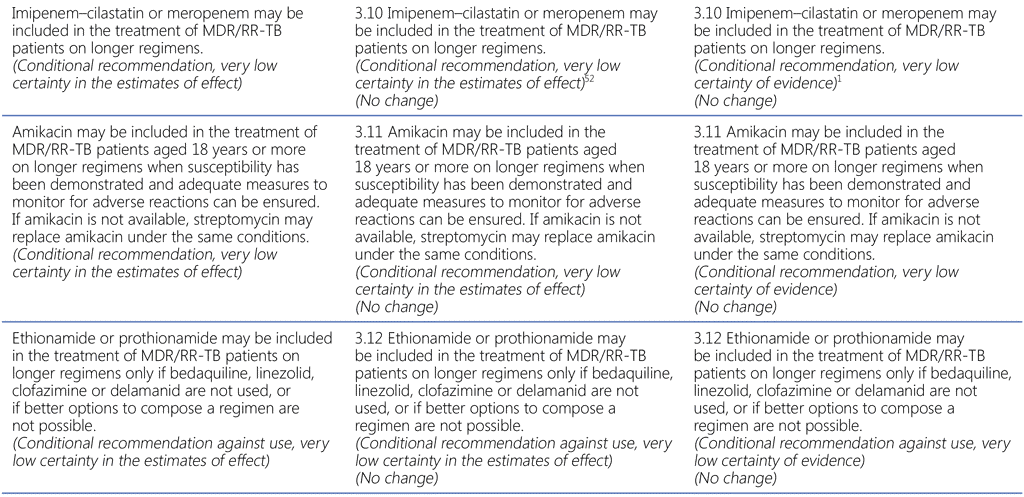
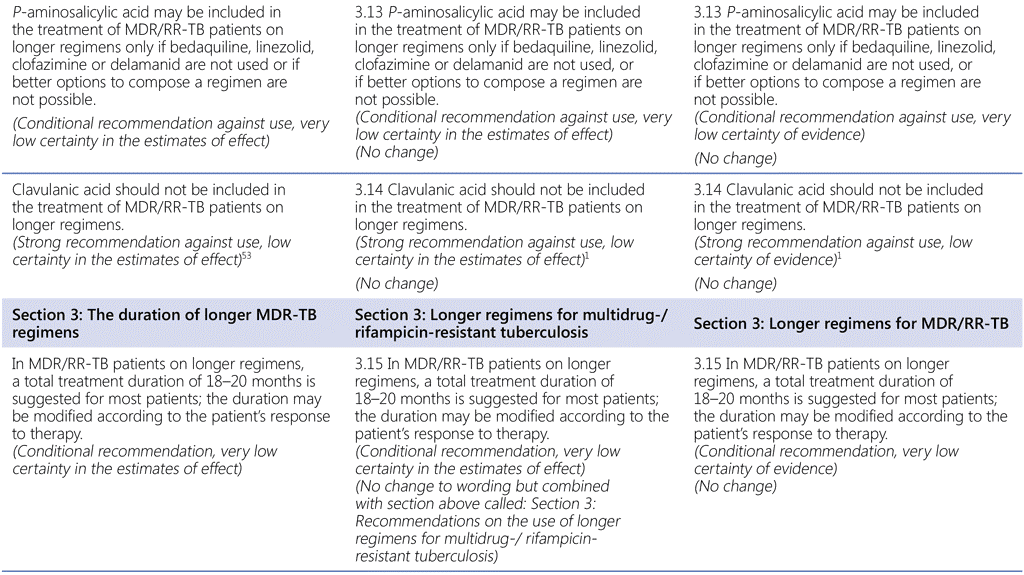
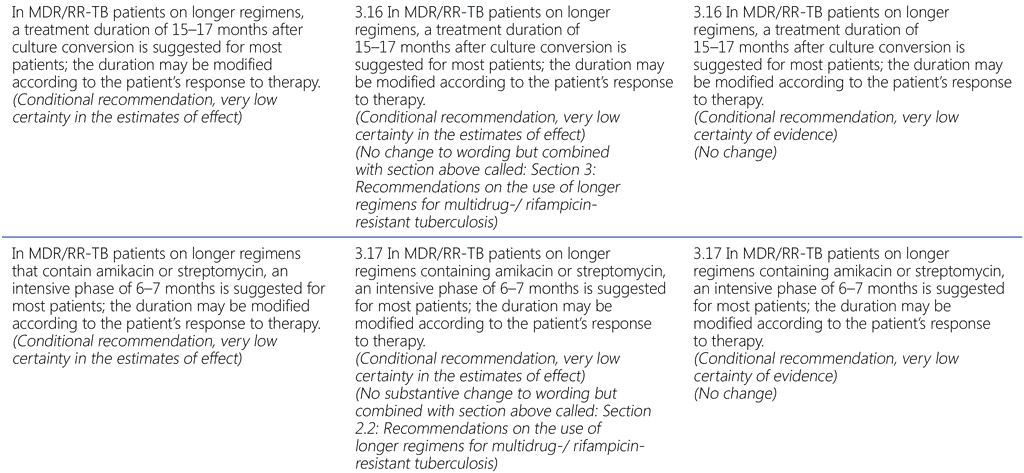
Summary of changes to the World Health Organization (WHO) treatment recommendations for multidrug-resistant or rifampicin-resistant TB (MDR/RR-TB) between 2019, 2020 and the current update (in 2022).
Notes:
- The WHO consolidated guidelines on drug-resistant tuberculosis treatment are a collection of currently valid and new recommendations on the treatment and management of MDR/RR-TB; as such, they include new recommendations developed in 2022 and all valid recommendations that had been previously published.
- In the current update (2022), there are two new recommendations (Recommendations 1.1 and 2.1). Recommendation 1.1 is a development based on new evidence on the bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM)/bedaquiline, pretomanid and linezolid (BPaL) regimen and Recommendation 2.1 is an update to a previous recommendation on shorter regimens for MDR/RR-TB.
- Recommendations on the use of bedaquiline and delamanid in children aged below 3 and 6 years were added from the WHO consolidated guidelines on tuberculosis. Module 5. Management of tuberculosis in children and adolescents.
- Recommendations on TB care and support are grouped in a separate submodule of the WHO consolidated guidelines on tuberculosis. Module 4. Treatment.
- All other recommendations remain unchanged.
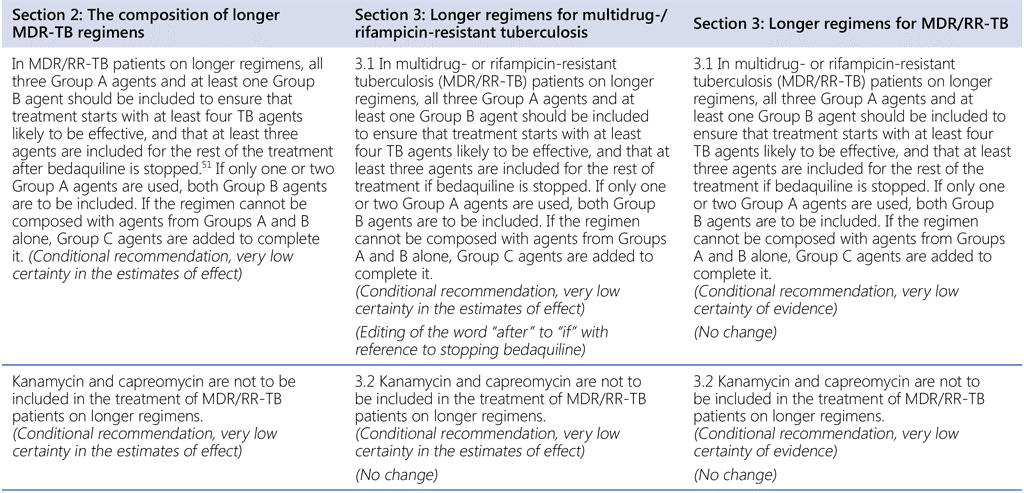
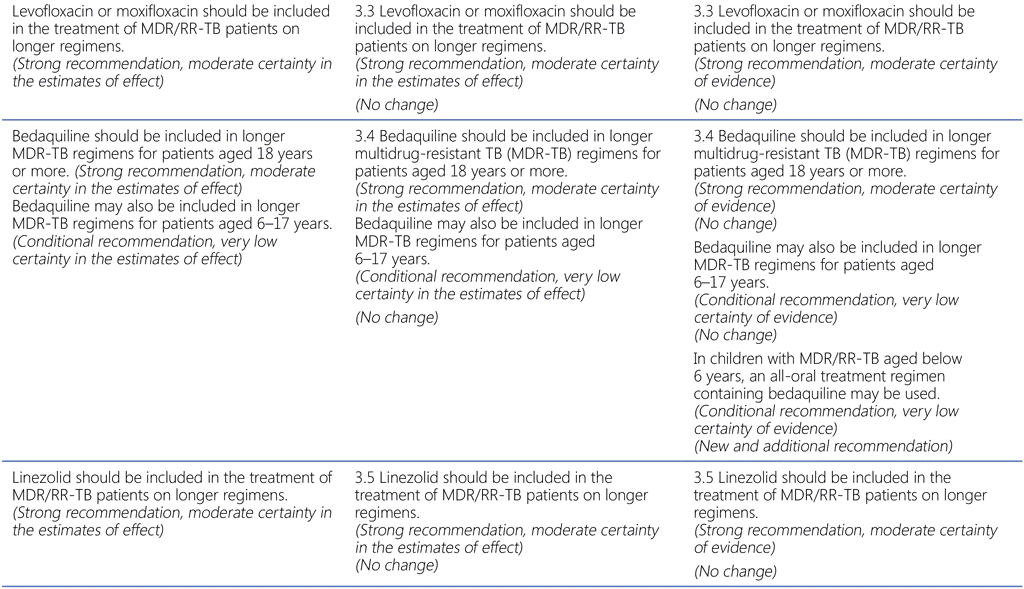
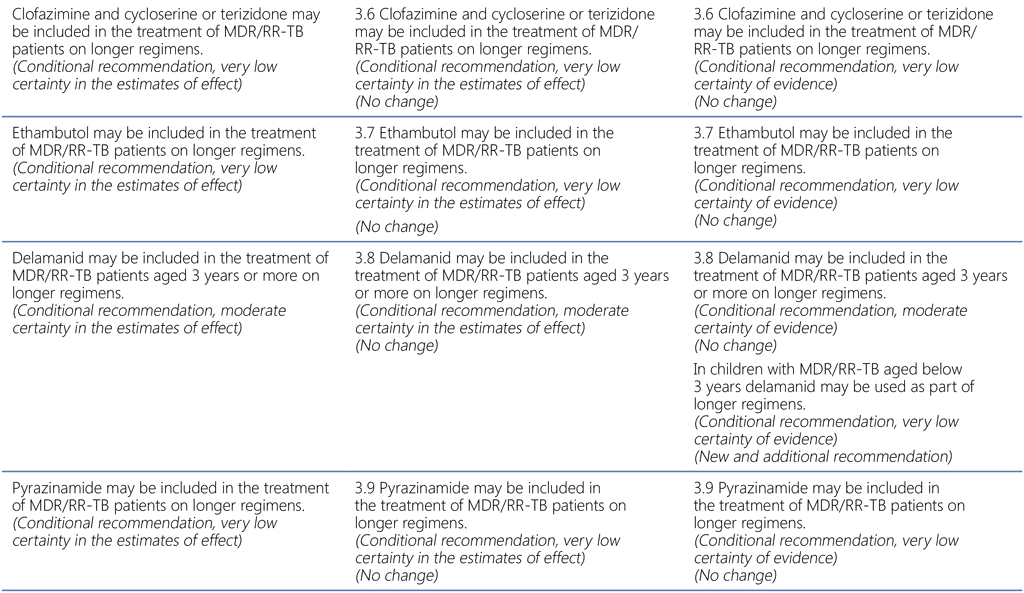
51 Group A = levofloxacin/moxifloxacin, bedaquiline, linezolid; Group B = clofazimine, cycloserine/terizidone; Group C = ethambutol, delamanid, pyrazinamide, imipenem–cilastatin, meropenem, amikacin (streptomycin), ethionamide/prothionamide, p-aminosalicylic acid (see also Table 3.1).
52 Imipenem–cilastatin and meropenem are administered with clavulanic acid, which is available only in formulations combined with amoxicillin. Amoxicillin–clavulanic acid is not counted as an additional effective TB agent, and should not be used without imipenem– cilastatin or meropenem.
53 Imipenem–cilastatin and meropenem are administered with clavulanic acid, which is available only in formulations combined with amoxicillin (amoxicillin–clavulanic acid). When included, clavulanic acid is not counted as an additional effective TB agent and should not be used without imipenem–cilastatin or meropenem.
 Feedback
Feedback