Book traversal links for Lateral flow urine lipoarabinomannan assay
Tests based on the detection of the lipoarabinomannan (LAM) antigen in urine have emerged as potential point-of-care tests for TB. The currently available urinary LAM assays have suboptimal sensitivity, and are therefore not suitable as general diagnostic tests for TB. However, unlike traditional diagnostic methods, they demonstrate improved sensitivity for the diagnosis of TB among individuals coinfected with HIV. The estimated sensitivity is even greater in patients with low CD4 cell counts. The lateral flow urine LAM assay (LF-LAM) strip-test – the Alere Determine TB LAM Ag (USA), hereafter referred to as AlereLAM – is currently the only commercially available urinary LAM test that potentially could be used as a rule-in test for TB in patients with advanced HIV-induced immunosuppression, and facilitate the early initiation of anti-TB treatment.
Recommendations
In inpatient settings
94.2WHO strongly recommends using LF-LAM to assist in the diagnosis of active TB in HIV-positive adults, adolescents and children:
- with signs and symptoms of TB (pulmonary and/or extrapulmonary)
(strong recommendation, moderate certainty in the evidence about the intervention effects); or - with advanced HIV disease¹⁵ or who are seriously ill¹⁶
(strong recommendation, moderate certainty in the evidence about the intervention effects); or - irrespective of signs and symptoms of TB and with a CD4 cell count of less than 200 cells/mm³
(strong recommendation, moderate certainty in the evidence about the intervention effects).
In outpatient settings
94.2WHO suggests using LF-LAM to assist in the diagnosis of active TB in HIV-positive adults, adolescents and children:
- with signs and symptoms of TB (pulmonary and/or extrapulmonary) or seriously ill
(conditional recommendation, low certainty in the evidence about test accuracy); and - irrespective of signs and symptoms of TB and with a CD4 cell count of less than 100 cells/mm³
(conditional recommendation, very low certainty in the evidence about test accuracy).
In outpatient settings
94.2WHO recommends against using LF-LAM to assist in the diagnosis of active TB in HIV-positive adults, adolescents and children:
- without assessing TB symptoms
(strong recommendation, very low certainty in the evidence about test accuracy); - without TB symptoms and unknown CD4 cell count or without TB symptoms and CD4 cell count greater than or equal to 200 cells/mm³
(strong recommendation, very low certainty in the evidence about test accuracy); and - without TB symptoms and with a CD4 cell count of 100–200 cells/mm³
(conditional recommendation, very low certainty in the evidence about test accuracy).
Remarks
- The reviewed evidence and recommendations apply to the use of AlereLAM only, because other in-house LAM-based assays have not been adequately validated or used outside limited research settings. Any new or generic LAM-based assay should be subject to adequate validation in the settings of intended use.
- All patients with signs and symptoms of pulmonary TB who are capable of producing sputum should submit at least one sputum specimen for Xpert MTB/RIF (Ultra) assay, as their initial diagnostic test. This also includes children and adolescents living with HIV who are able to provide a sputum sample.
- These recommendations also apply to adolescents and children living with HIV, based on generalization of data from adults, while acknowledging that there are very limited data for these population groups.
- LF-LAM should be used as an add-on to clinical judgement in combination with other tests; it should not be used as a replacement or triage test.
Test description
The urine-based LF-LAM AlereLAM is a commercially available point-of-care test for active TB (24). AlereLAM is an immunocapture assay that detects LAM antigen in urine, LAM being a lipopolysaccharide present in mycobacterial cell walls released from metabolically active or degenerating bacterial cells during TB disease (24, 25).
AlereLAM is performed manually by applying 60 µL of urine to the test strip (the white pad marked by the arrow symbols in Fig. 2.2.3 A) and incubating at room temperature for 25 minutes. The strip is then inspected by eye for visible bands. The intensity of any visible band on the test strip is graded by comparing it with the intensities of the bands on a manufacturer-supplied reference scale card (as shown in the example in Fig. 2.2.3 B).
Fig. 2.2.3. Alere Determine TB LAM Ag tests (AlereLAM): (a) individual test strip, and (b) reference card accompanying test strips to “grade” the test result and determine positivity
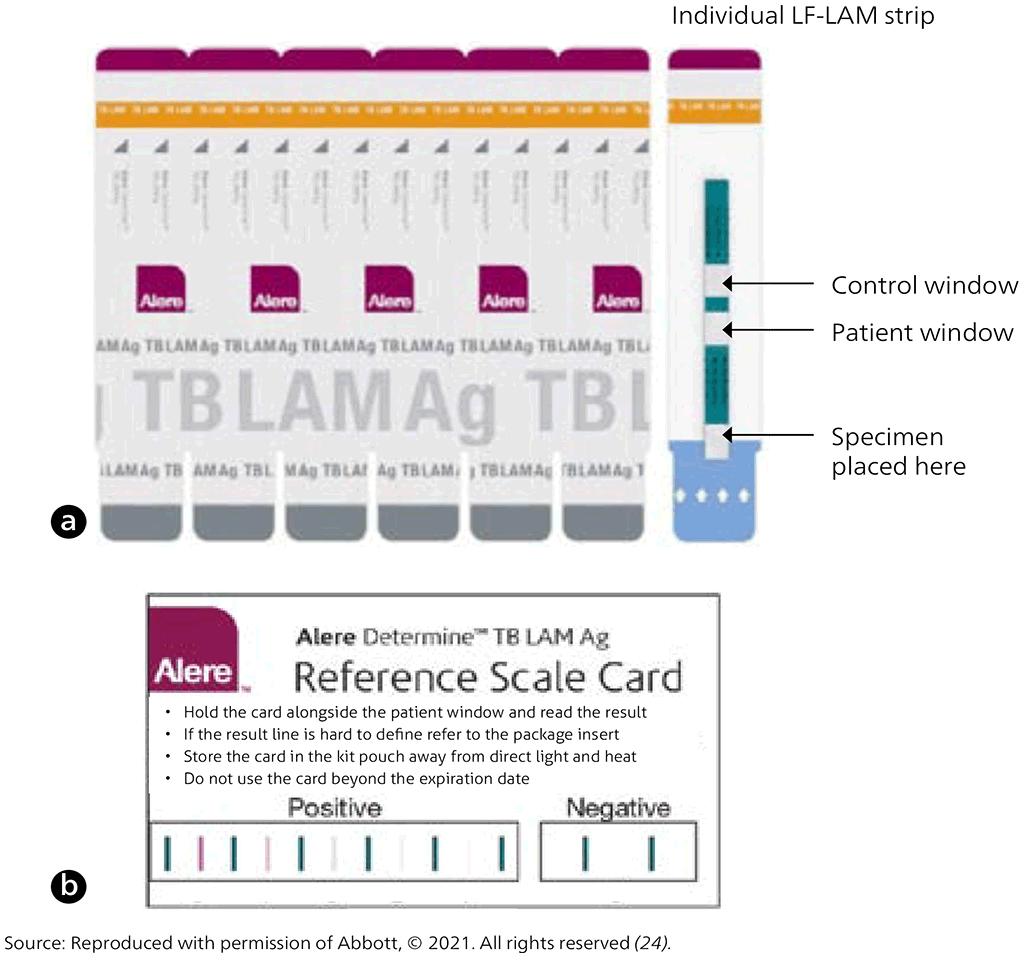
AlereLAM is being considered as a diagnostic test that may be used in combination with existing tests for the diagnosis of HIV-associated TB.
Justification and evidence
WHO commissioned a systematic review to summarize the current scientific literature on the accuracy of AlereLAM for the diagnosis of TB in PLHIV as part of a WHO process to develop updated guidelines for the use of the AlereLAM assay.
The PICO questions shown in Box 2.2.2 were designed to form the basis for the evidence search, retrieval and analysis.

The review identified 15 unique published studies that assessed the accuracy of AlereLAM in adults, and integrated nine new studies identified since the original WHO and Cochrane reviews in 2015 and 2016, respectively (26, 27). All studies included in the systematic review were performed in high TB/HIV burden countries. The positive AlereLAM results were reported in accordance with the manufacturer’s updated recommendations for test interpretation (graded on a scale of 1 to 4, based on band intensity). All analyses were performed with respect to an MRS.
The 15 included studies involved 6814 participants, of whom 1761 (26%) had TB. Eight of the studies evaluated the accuracy of AlereLAM for TB diagnosis in participants with signs and symptoms suggestive of TB; these studies involved 3449 participants, of whom 1277 (37%) had TB. Seven studies evaluated the accuracy of AlereLAM for diagnosis of unselected participants who may or may not have had TB signs and symptoms at enrolment; these studies involved 3365 participants, of whom 439 (13%) had TB.
All studies were performed in high TB/HIV burden countries that were classified as low-income or middle-income countries. The studies had substantial differences in the following characteristics: study population (“studies with symptomatic participants” and “studies with unselected participants”), setting (inpatients versus outpatients), median CD4 cell count, TB prevalence, inclusion and exclusion of participants based on whether or not they could produce sputum, and whether patients were evaluated for pulmonary TB or extrapulmonary TB, or both.
Most studies reported that a valid AlereLAM result was obtained on the first attempt for all tests. Uninterpretable test results (<1%) were reported in only three studies (28–30).
Summary of the results
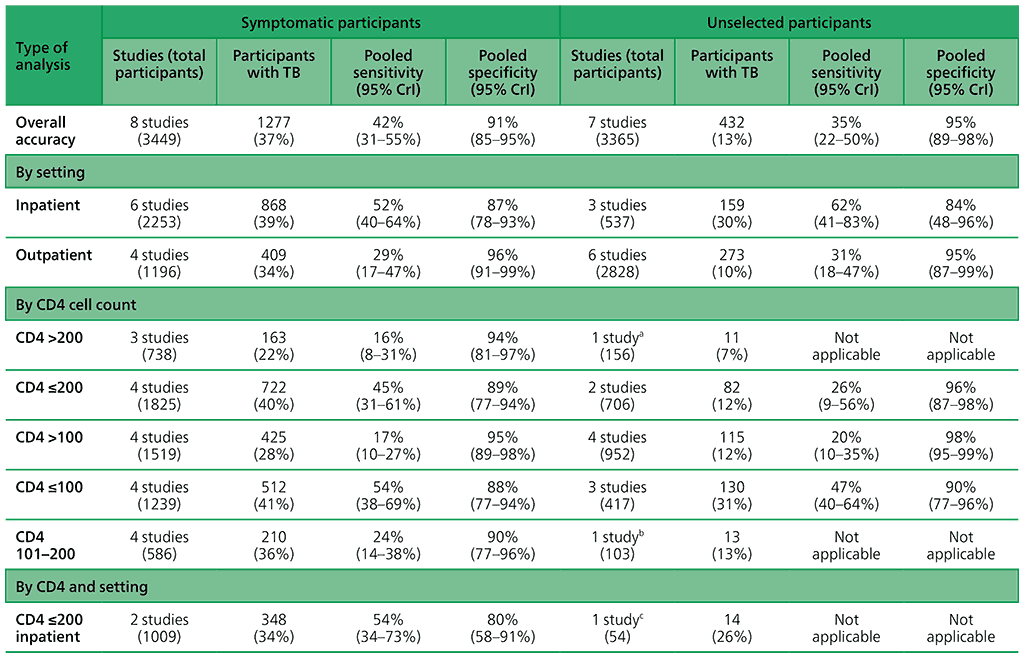
For TB diagnosis in HIV-positive adults presenting with signs and symptoms of TB, the diagnostic accuracy of AlereLAM is as follows:
- in inpatient settings, sensitivity 52% (40–64%)¹⁷ and specificity 87% (78–93%);
- in outpatient settings, sensitivity 29% (17–47%) and specificity 96% (91–99%); and
- in all settings, sensitivity 42% (31–55%) and specificity 91% (85–95%).
For TB diagnosis in HIV-positive adults, irrespective of signs and symptoms of TB, the diagnostic accuracy of AlereLAM is as follows:
- in inpatient settings, sensitivity 62% (41–83%) and specificity 84% (48–96%);
- in outpatient settings, sensitivity 31% (18–47%) and specificity 95% (87–99%); and
- in all settings, sensitivity 35% (22–50%) and specificity 95% (89–98%).
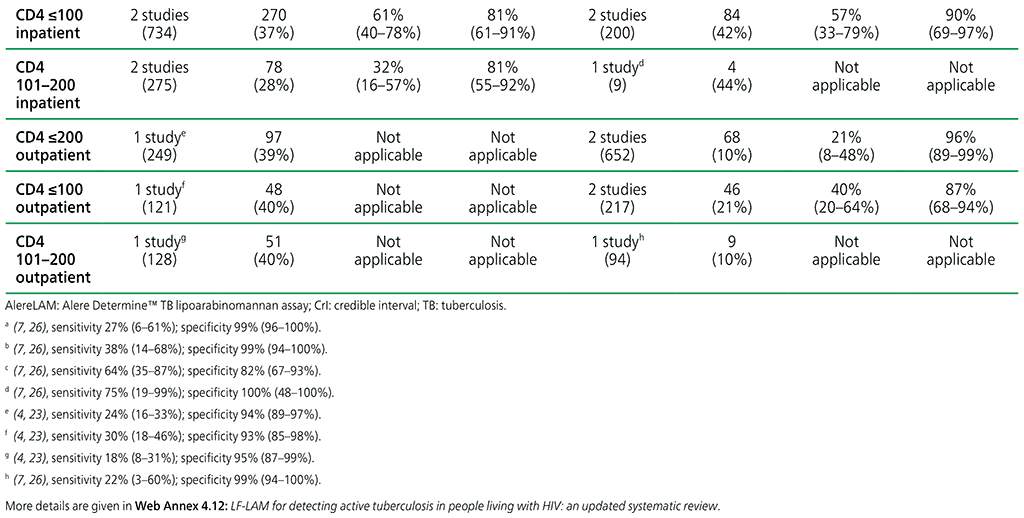
For diagnosis of TB in adults with advanced HIV disease, irrespective of signs and symptoms of TB, the diagnostic accuracy of AlereLAM (limited data available) is as follows:
- in inpatient settings, CD4 cell count ≤200, sensitivity 64% (35–87%) and specificity 82% (67– 93%) (one study);
- in outpatient settings, CD4 cell count ≤200, sensitivity 21% (8–48%) and specificity 96% (89–99%);
- in all settings, CD4 cell count ≤200, sensitivity 26% (9–56%) and specificity 96% (87–98%);
- in inpatient settings, CD4 cell count ≤100, sensitivity 57% (33–79%) and specificity 90% (69–97%);
- in outpatient settings, CD4 cell count ≤100, sensitivity 40% (20–64%) and specificity 87% (68–94%); and
- in all settings, CD4 cell count ≤100, sensitivity 47% (30–64%) and specificity 90% (77–96%).
For diagnosis of TB in HIV-positive children, the diagnostic accuracy of AlereLAM (limited data available) is as follows:
- in all settings, including all children, for individual studies, sensitivity and specificity were:
- 42% (15–72%) and 94% (73–100%) (one study conducted in an outpatient setting);
- 56% (21–86%) and 95% (90–98%) (one study conducted in an inpatient setting); and
- 43% (23–66%) and 80% (69–88%) (one study conducted in both inpatient and outpatient settings).
For use of AlereLAM to reduce mortality associated with advanced HIV disease (two randomized trials):
- the pooled risk ratio for mortality was 0.85 (0.76–0.94); and
- the absolute effect was 35 fewer deaths per 1000 (from 14 fewer to 55 fewer) (PICO 4).
Table 2.2.2 presents pooled sensitivity and specificity results for AlereLAM against an MRS grouped by the study population, TB diagnosis among “symptomatic participants” and TB diagnosis among “unselected participants”.
Table 2.2.2. AlereLAM pooled sensitivity and specificity for TB diagnosis, by study population
Cost–effectiveness analysis
Economic evidence for the implementation and scale-up of LF-LAM is limited. The studies that have been done show a consistent trend, suggesting that LF-LAM could be cost effective in a population of African adults living with HIV (particularly among hospitalized patients).
More details are given in Web Annex 4.13: Economic evaluations of LF-LAM for the diagnosis of active tuberculosis in HIV-positive individuals: an updated systematic review.
User perspective
For a qualitative study on user perspectives, 15 semi-structured interviews were conducted during February and March 2019 with clinicians, nurses, programme officers, laboratory staff and patient advocates in Kenya, South Africa and Uganda. The results showed that LF-LAM clearly addresses a need and makes an important difference in a population in which TB is hard to diagnose. In line with the global discourse on LF-LAM, the participants in this study generally saw LF-LAM as an easy-to-use, rapid test that requires little maintenance and equipment, and crucially does not rely on sputum but on urine, a specimen that is safer to work with and easier to obtain. However, the perceived benefits of the specimen, turnaround time, user-friendliness, cost and maintenance requirements can also pose a challenge, depending on the particular situation and the capacities in which the test is used. Similarly, the infrastructure requirements are minimal but there can still be challenges with stock-outs, lack of urine containers and shelf life. Finally, even though the turnaround time is in theory only 25 minutes, in many settings, treatment is not initiated until the next day.
Overall, the results from the qualitative study suggest that the benefits outweigh the challenges, especially given the absence of viable diagnostic alternatives for this particular patient group. These results also show that it is essential to pay attention to how diagnostics are operationalized. Just because a technology is quicker, easier to conduct and cheaper than existing diagnostics, this does not mean it is necessarily more successful in being implemented.
More details are given in Web Annex 4.14: User perspectives on TB-LAM for the diagnosis of active tuberculosis: results from qualitative research.
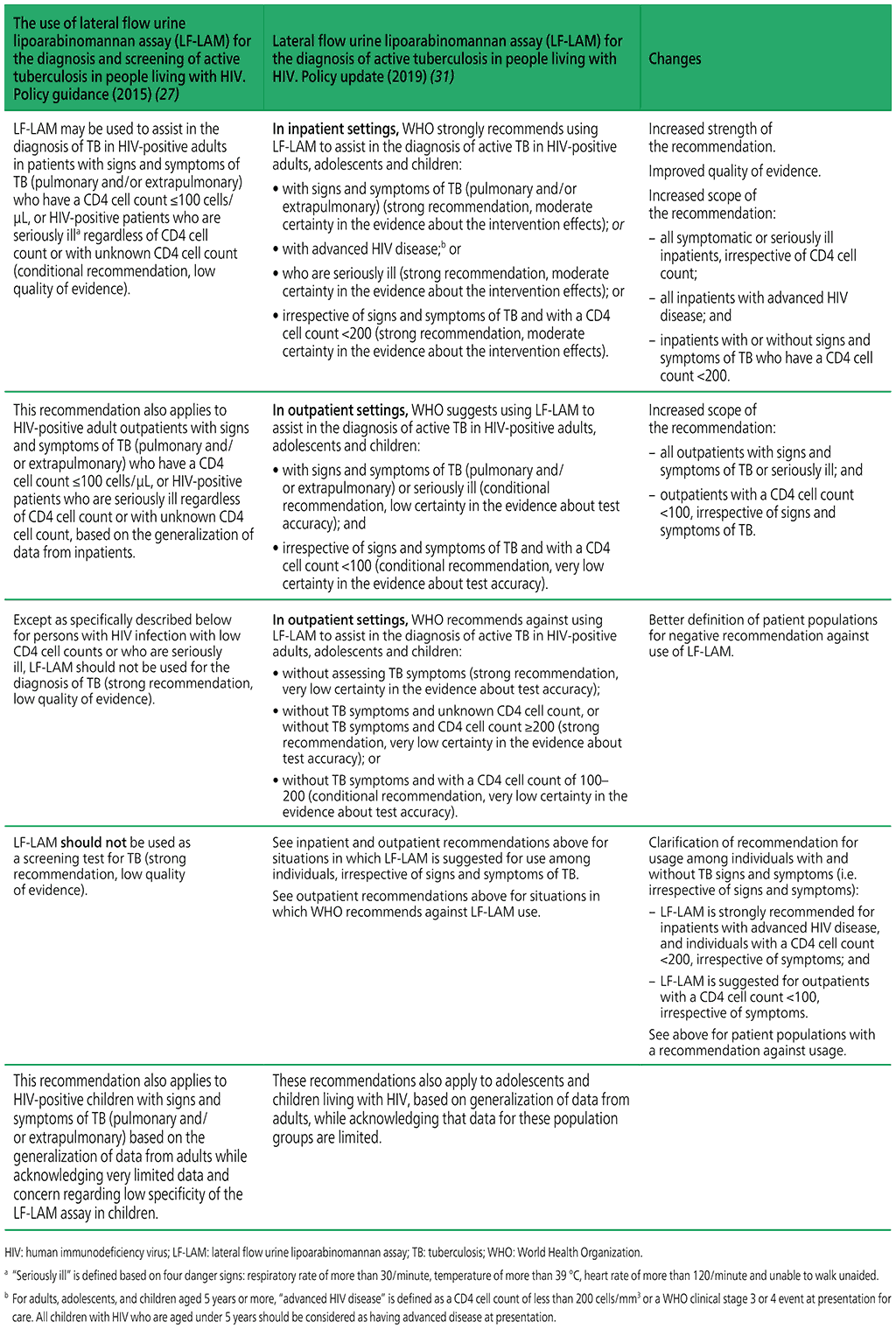
Summary of changes between the 2015 guidance and the 2019 update
Research priorities
- Development of simple, more accurate tests based on LAM detection, with the potential to be used for HIV-negative populations.
- Evaluation of the use of LF-LAM in PLHIV without signs and symptoms of TB.
- Evaluation of the use of LF-LAM in children and adolescents with HIV.
- Evaluation of the combination of parallel use of LF-LAM and rapid qualitative CD4 cell count systems.
- Undertaking of implementation research into the acceptance, scale-up and impact of LF-LAM in routine clinical settings.
- Undertaking of qualitative research on user perspectives of LF-LAM for feasibility, accessibility and equity issues.
- Undertaking of implementation research on LF-LAM integrated into HIV care packages.
- Evaluation of the performance of LF-LAM as the HIV epidemic evolves and more people on treatment with viral load suppression are hospitalized.
- Evaluation of the cost–effectiveness of LF-LAM.
- Evaluation of other rapid LAM-based tests such as FujiLAM.
15 For adults, adolescents, and children aged 5 years or more, “advanced HIV disease” is defined as a CD4 cell count of less than 200 cells/mm3 or a WHO clinical stage 3 or 4 event at presentation for care. All children with HIV aged under 5 years should be considered as having advanced disease at presentation.
16 "Seriously ill" is defined based on four danger signs: respiratory rate of more than 30/minute, temperature of more than 39 °C, heart rate of more than 120/minute and unable to walk unaided.
17 The numbers in brackets show the 95% credible interval (CrI).
 Feedback
Feedback