Book traversal links for 4.1 Considerations in selecting and using CAD for screening in TB programmes
CAD technologies for automated reading of digital CXR for TB detection offer a promising solution for high-TB burden countries; however, selecting the appropriate CAD product for a particular setting can be complex. When selecting a CAD product, TB programmes and implementers should consider multiple aspects of the technology and its interface with existing infrastructure, including:
- national and international regulatory approval of the products;
- the accuracy of the product for detecting abnormalities consistent with TB;
- the requirements for running the CAD programme, including:
- the requisite software and hardware. Most CAD products can be run on any digital radiography platform, but not all. This consideration should include options for integration with existing and legacy systems.
- the Internet connectivity required to run the programme. Although online deployment is the most common method of implementation, access to a stable Internet connection may be difficult. “Cloud-based” CAD programmes require stable Internet to function, and most CAD developers offer offline solutions that can be operated independently of Internet connection, although the cost may vary.
- the cost of running CAD. Pricing schemes for CAD programmes vary widely according to factors such as: bundled costs (whether hardware will be purchased with software), the costs of installation and set-up and the costs of reading. Each automated reading may be priced per CXR (usually volume) or structured as a set price for unlimited use for a certain time. Maintenance costs should also be considered.
- data security and privacy. Commercial cloud servers are used for online deployment by default. Countries are, however, increasingly concerned about privacy and request that servers be set up within their borders. Local or in-country servers could be set up, although at additional cost. When CXRs are analysed via commercial cloud servers, images will have to be anonymized before uploading. Most CAD solutions include an anonymization tool.
The market of CAD products for TB detection is constantly changing and expanding, with new versions of products and new companies coming online almost every day. FIND and the Stop TB Partnership have jointly created an online data repository of the CAD products currently available on the market and their key characteristics as described above, based on results of surveys with developers, which can be found at https://www.ai4hlth.org/. The goal of this publicly accessible, regularly updated, living database is to enable implementers to keep abreast of the rapidly changing artificial intelligence landscape.
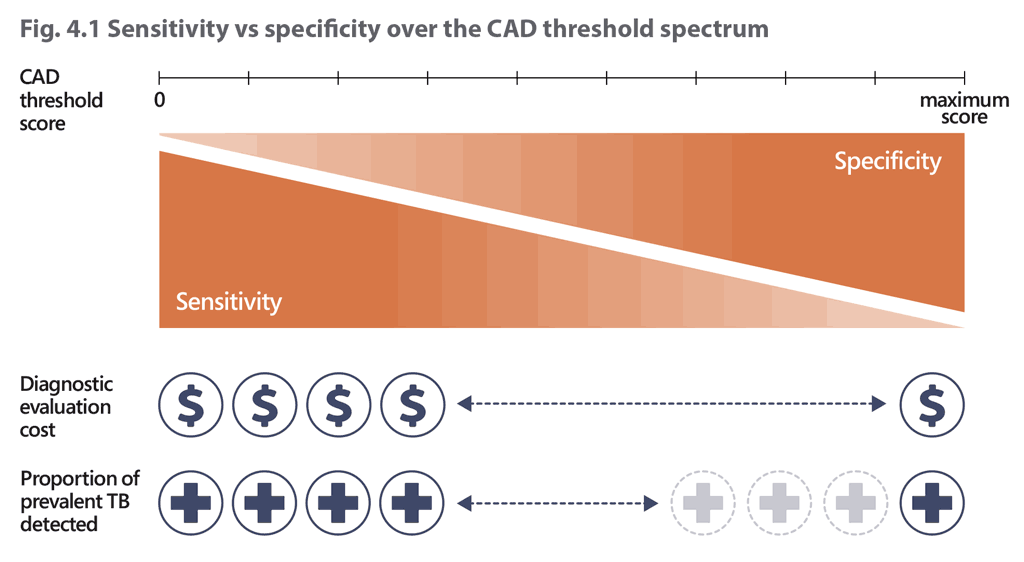
Effective integration of CAD products into routine TB screening or triage requires determination of an appropriate CAD threshold that will be used to signal a positive screen and trigger further TB diagnostic evaluation. As threshold values for CAD products are not static or consistent across software or even versions of the same software, the user must determine the most appropriate abnormality or threshold score for their setting and use case, above which a confirmatory diagnostic test would be conducted. Identifying the ideal threshold for each use of CAD requires decisions on the goals and acceptable costs of screening. As with other screening tools, there is an inherent trade-off in the selection of the threshold score; lower scores will maximize the sensitivity of the tool to detect true TB patients in the population being screened but will incur additional costs for diagnostic testing because of reduced specificity. Higher scores will reduce the volume, and thus costs, of diagnostic testing and will probably focus case detection on more severe cases, but the reduced sensitivity will result in missed cases (Fig. 4.1).
CAD can be integrated into TB screening or triage when human interpretation is not available, or it can be used with trained readers to reduce the workload.
- CAD can be used for initial screening, with any abnormal result referred to a human reader for final reading.
- CAD can be used for initial screening, with a portion of all results verified by a human reader (e.g.all abnormal and 10% of normal CAD results).
- CAD can entirely replace a human reader, with all abnormal results referred for diagnostic evaluation.
- CAD and human reading can be done in parallel, with an abnormal reading from either reading referred for diagnostic evaluation.
The performance of CAD in terms of the sensitivity and specificity of a particular threshold score are likely to depend on TB epidemiology and subgroups, such as people living with HIV and older people. Threshold scores also differ substantially by CAD product and even among versions of the same software. Determination of appropriate threshold scores based on local realities is therefore an integral part of the set-up and use of CAD and requires that TB programmes consider the overall aim of CAD in TB screening and diagnostic algorithms.
 Feedback
Feedback