Book traversal links for Annex 6. Variables to be collected for TB contact evaluation
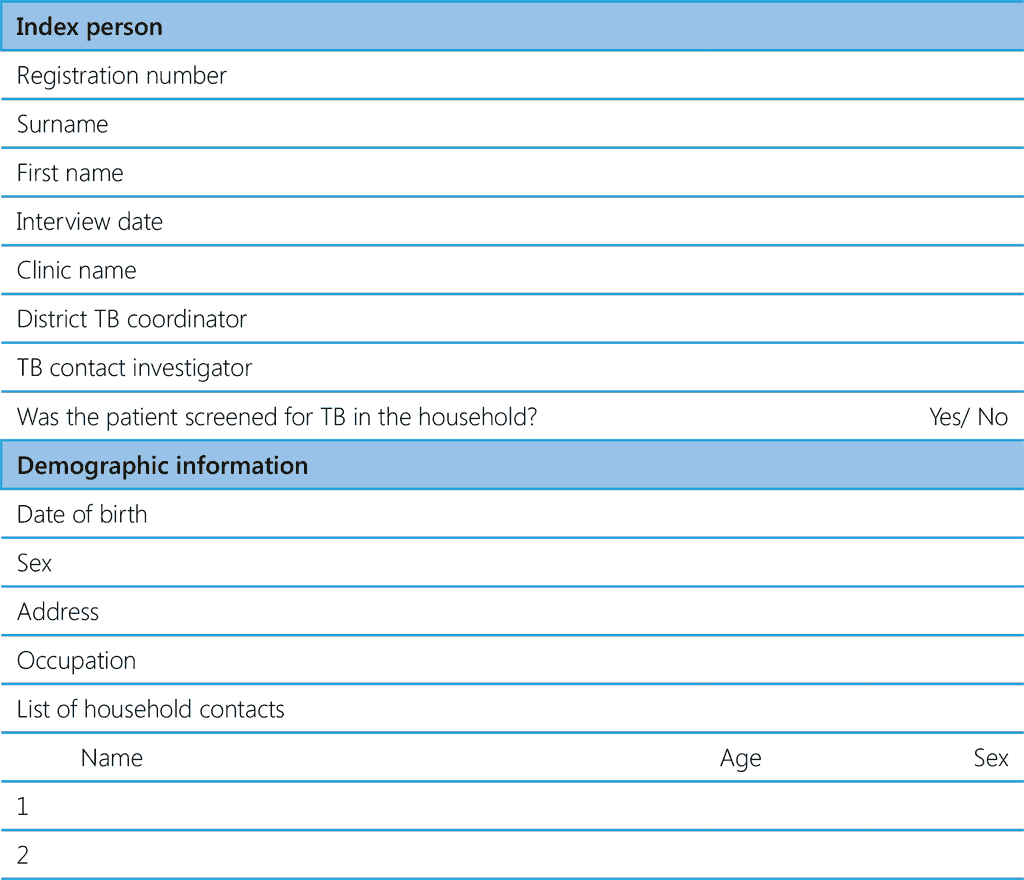
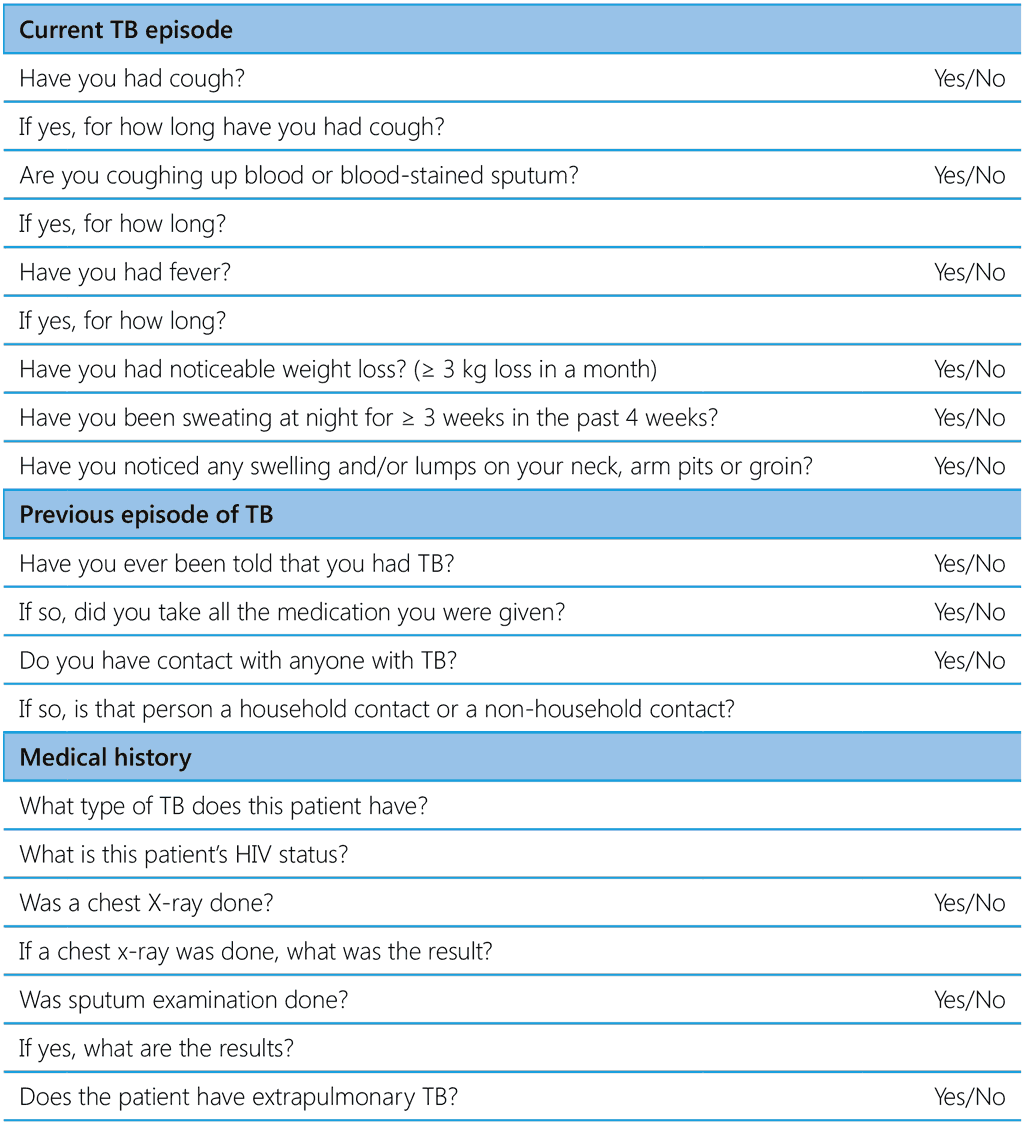
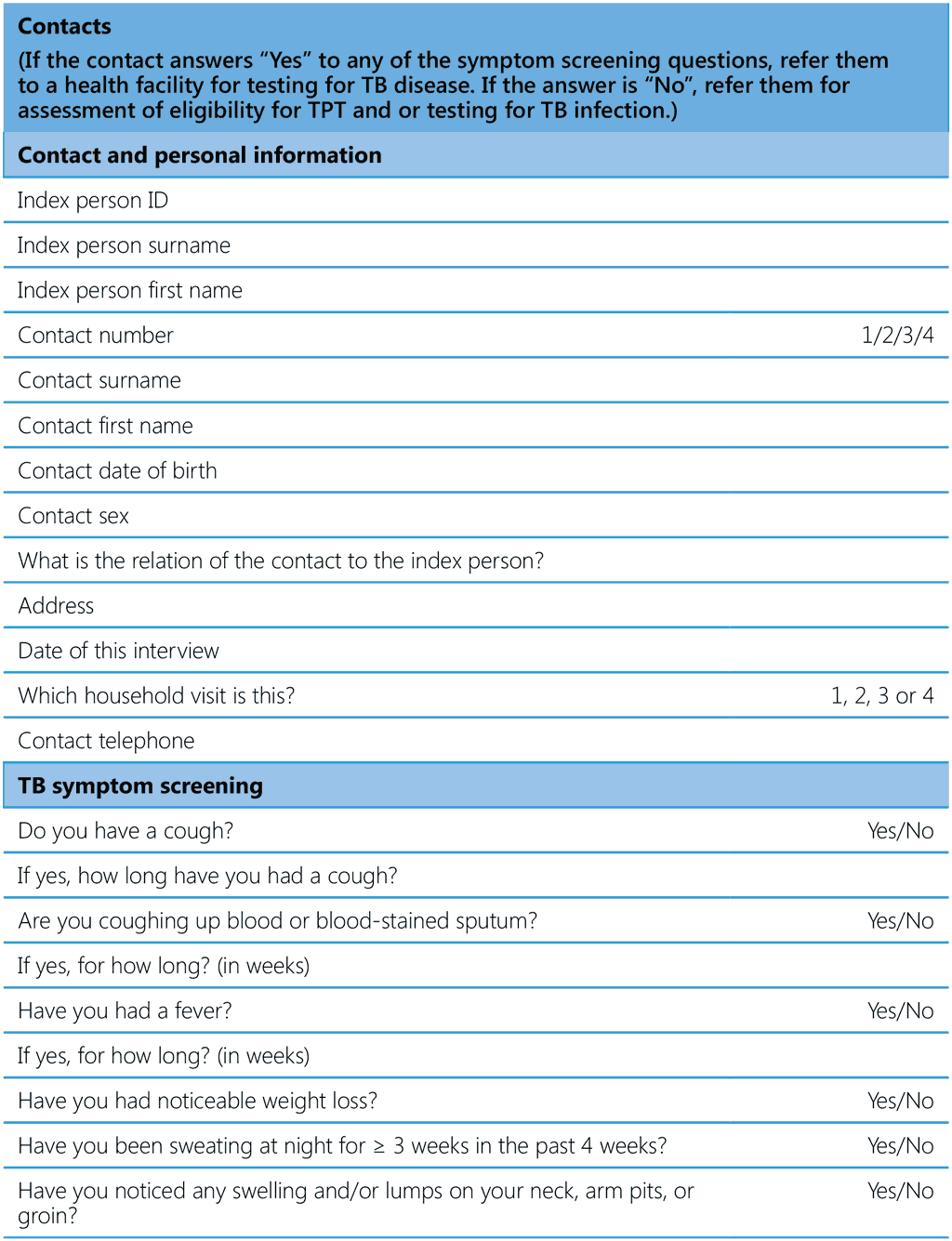
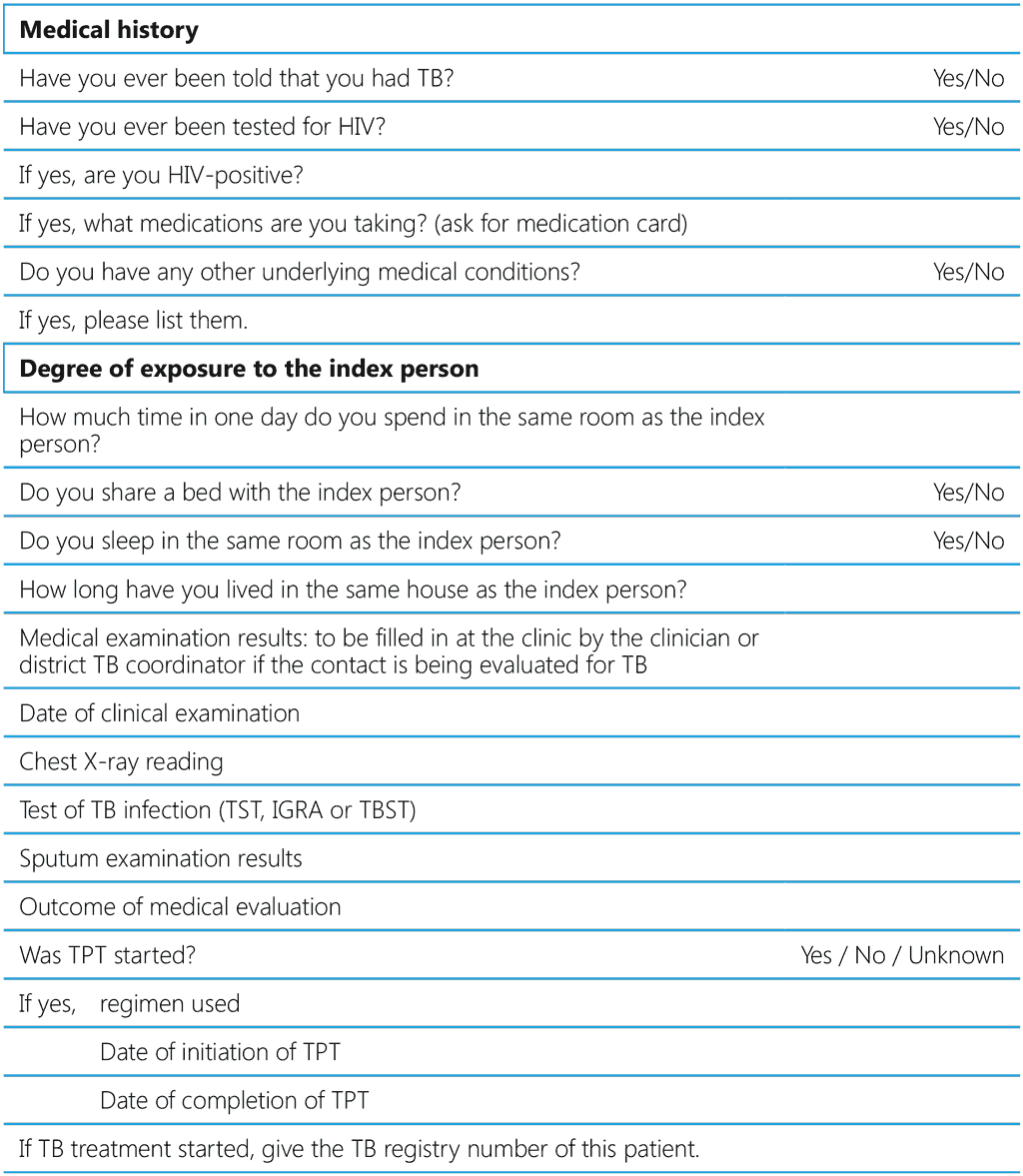
Table A6.1 proposes a list of variables on which data should be collected for index cases and their contacts in an evaluation of the contacts of a person with TB disease. The data may be collected at various stages of the investigation. Usually, demographic information and much of a person’s medical history is available at the first visit, while other details, such as the results of tests for ruling out TB, confirming infection or starting TPT would be collected at subsequent encounters. Ideally, the data should be registered electronically to facilitate retrieval, storage and analysis.
Table A6.1 Suggested data variables for capture at different steps of contact evaluation
 Feedback
Feedback