Book traversal links for 6.2.2 Medicines used in longer MDR-TB treatment regimens
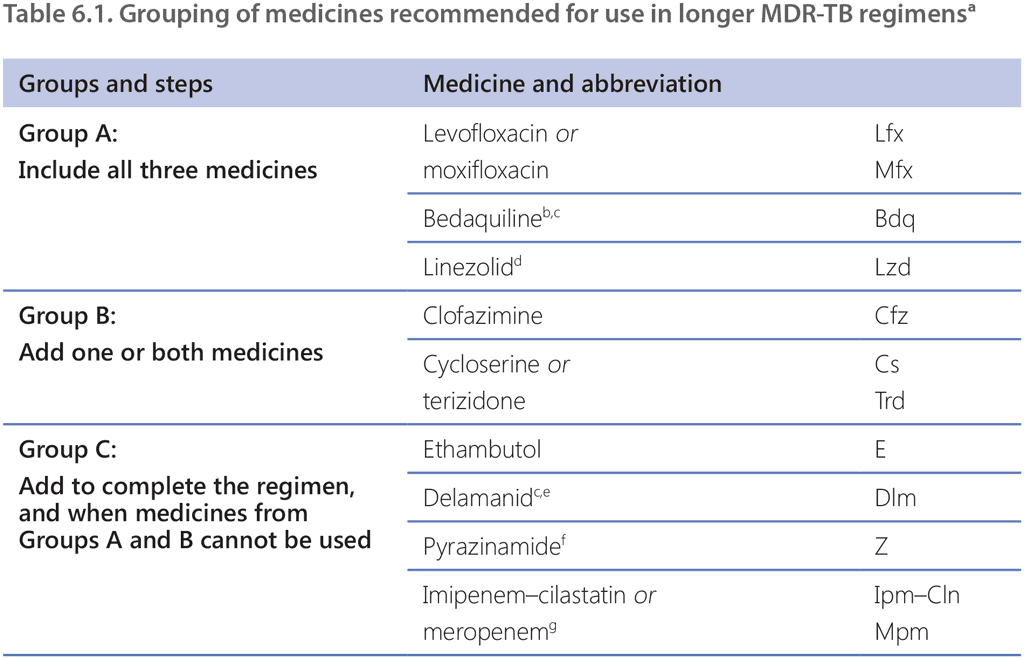
The classification of medicines used in MDR/RR-TB treatment regimens was revised following the evidence-informed update of the WHO guidelines on DR-TB treatment in 2018. TB medicines to be used for treatment of MDR/RR-TB are categorized into Groups A, B and C (Table 6.1) (1). This classification is based on drug class and level of certainty in the evidence on effectiveness and safety (i.e. balance between benefits and risk of harm). The data analysed relate mainly to adult patients who received regimens in recent years. Groups A–C feature the medicines to be used to compose longer MDR-TB regimens. WHO considers that, under programmatic conditions, only these medicines (Groups A–C) have a role in longer MDR-TB treatment regimens. In addition to agents from Groups A–C, the potential role for clavulanic acid and high-dose isoniazid was discussed (see “Other medicines” in this section).
The most notable differences between the classification of longer regimen components used before 2018 and the current guidelines are an upgrade in the priority of bedaquiline, linezolid, clofazimine and cycloserine/terizidone; placement of delamanid in Group C; and lowering of priority for pyrazinamide, amikacin, streptomycin, ethionamide/prothionamide and p-aminosalicylic acid, relative to other treatment options. Several agents that were featured previously in these groups are no longer included because they are:
- no longer recommended (e.g. ofloxacin, capreomycin and kanamycin);
- rarely used in longer regimens (e.g. high-dose isoniazid); or
- an adjunct agent that is not intended to be used alone (e.g. clavulanic acid is used only in combination with the carbapenems).
The classification facilitates design of the treatment regimen for patients with DR-TB who are not eligible for the BPaLM/BPaL or 9-month treatment regimens. Table 6.1 summarizes the general steps to take when including agents for the longer MDR-TB regimen according to the latest WHO guidance, with more details provided for some of the most common situations and patient subgroups that clinicians and NTPs may encounter.
Group A
Group A includes fluoroquinolones (levofloxacin and moxifloxacin), bedaquiline and linezolid. These medicines were found to be highly effective in improving treatment outcomes and reducing deaths in the evidence reviewed in 2018 for the WHO guidelines (1), and it is strongly recommended that they be included in all longer MDR-TB regimens and used for all MDR/RR-TB patients eligible for longer regimens unless there is a toxicity issue or drug resistance.
Levofloxacin and moxifloxacin
Levofloxacin and moxifloxacin are later-generation fluoroquinolones, and their use in the meta-analysis that informed the WHO guidelines (2018 update) resulted in a significantly lower risk of treatment failure or relapse and death (1, 11, 68, 69). Levofloxacin and moxifloxacin appear to be equally effective in fluoroquinolone-sensitive patients receiving longer regimens, and either of these drugs can be considered for MDR/RR-TB treatment using these regimens. Ciprofloxacin and ofloxacin are less effective in MDR-TB treatment and are no longer recommended.
Reliable rapid molecular DST is available for levofloxacin and moxifloxacin (including Xpert MTB/ XDR and second-line LPA). Not all point mutations present the same resistance profile. Despite some mutations having consistently high minimum inhibitory concentrations (MICs) (i.e. gyrA D94N or D94Y), most mutations present a range of phenotypic resistance that may cross critical concentration (CC) and clinical breakpoint (CB) levels. Therefore, once fluoroquinolone resistance has been detected by molecular methods and treatment has started, a phenotypic method may be used as a reference test for distinguishing between high-level (>CB) and low-level (>CC and <CB) resistance mutations, possibly allowing for the use of a high-level fluoroquinolone dose. Where these mutations are detected, the composition of the longer regimen should be re-evaluated based on phenotypic DST results at the CB (70).
If DST for moxifloxacin confirms high-level resistance, or if the patient’s history suggests that moxifloxacin has not been effective (e.g. if used in a failing regimen for more than 15–30 days), moxifloxacin should not be used. Work is ongoing to optimize the use of moxifloxacin related to sequencing, CC in phenotypic DST and clinical correlation (70–72).
Bedaquiline
In the IPD meta-analysis used as evidence for the WHO guidelines, bedaquiline use resulted in significantly fewer episodes of treatment failure, relapse and death (1). There is growing experience of its use in children, adolescents and older people, patients with extrapulmonary TB disease and People with HIV (73, 74). Currently, there is no age restriction for the use of bedaquiline, including in longer regimens (47).
Analyses of observational study data highlighted the improved survival of patients treated with regimens containing bedaquiline (47) and the favourable safety profile of bedaquiline when the drug is used alongside other TB medicines, including medicines with a QT prolongation effect (e.g. moxifloxacin, clofazimine and delamanid) (75–80). The recent data review for the WHO consolidated guidelines (1) suggested no additional safety concerns for the use of bedaquiline beyond 6 months, used concurrently with delamanid or in pregnancy (77). The available data suggested that the concurrent use of bedaquiline and delamanid does not increase the risk of clinically meaningful QT prolongation (81).
Some inconclusive evidence is emerging; for example, some published data on the rapid advent of bedaquiline resistance in settings where it is used may suggest a possibility of bedaquiline being a low genetic barrier drug (i.e. causing resistance to emerge rapidly) as a result of frequent natural mutations. Also worth considering is the long half-life of the drug (5.5 months), which may lead to the drug acting as monotherapy in patients lost to follow-up. Fluoroquinolone resistance testing should be performed to prevent bedaquiline resistance acquisition, and the levels of resistance should be monitored when possible. Bedaquiline presents cross-resistance with clofazimine in cases of Rv0678 gene mutation (which lead to upregulation of efflux pumps) and pepQ mutations. Resistance may occur spontaneously, even without prior exposure to bedaquiline or clofazimine (4.1% in some studies) (82, 83). Mutations at the atp-E gene may confer high-level resistance to bedaquiline.
Linezolid
Linezolid has shown anti-TB activity in vitro and in animal studies, and its effectiveness in humans was demonstrated in the meta-analysis conducted for the WHO guidelines, as well as in recent trials involving XDR-TB patients (1, 84–88).
Linezolid is associated with considerable toxicity, which necessitates close monitoring for signs of bone marrow suppression and neuropathies. The 2018 IPD meta-analysis informing the WHO guidelines included information from more than 300 patients who were treated with linezolid for at least 1 month, mostly on 600 mg daily. About 30% of patients received linezolid for 1–6 months, but over 30% received it for more than 18 months, and these patients had the lowest frequency of treatment failure, loss to follow-up and death. This analysis also suggested that the optimal duration of use would be about 20 months, corresponding to the usual total duration of a longer MDR-TB regimen; however, the analysis did not account for survivorship bias (i.e. that those who complete the full length of treatment are more likely to have a successful outcome, given that deaths and losses to follow-up occur earlier) (1, 89).
The evidence from the WHO consolidated guidelines (1) suggests that linezolid should be used for as long as it is tolerated. There may be improved outcomes if linezolid is used for the full duration of treatment. However, it probably has its greatest added effect (including protection of other secondline drugs against acquired drug resistance) during the first months of treatment when the bacillary load is highest (90). If toxicity develops, dosing of linezolid should be reduced or replaced by another bactericidal drug (17).
Linezolid is not affected or metabolized by the cytochrome p450; however, it is an inhibitor of monoamine oxidase (IMAO), leading to an increase in serotonin and tyramine levels. Serotonergic syndrome, which can be serious and life threatening, can result when linezolid is given concomitantly with other IMAO drugs that are often used in clinical practice in TB patients (e.g. antidepressants, opioid pain killers such as tramadol, common cold medications or antitussives such as dextromethorphan) (91).
Group B
Group B medicines include clofazimine and cycloserine or terizidone, which were found to be effective in improving treatment outcomes but limited in reducing deaths in the evidence reviewed in 2018 for the WHO guidelines (1). One or both drugs can be added to ensure that a longer regimen starts with at least four effective medicines.
Clofazimine
Clofazimine is an antileprosy medicine that has shown in vitro activity against M. tuberculosis and has been used as a second-line TB medicine for several years. The meta-analysis conducted for the WHO guidelines reinforced the evidence for the effectiveness and safety profile of clofazimine (1). When used with drugs that prolong the QT interval (e.g. bedaquiline, fluoroquinolones and delamanid), clofazimine may cause additive QT prolongation. ECG monitoring should be implemented when bedaquiline is used or when several QT-prolonging drugs are also part of the regimen. Non-TB drugs that cause QT prolongation should be avoided if possible.
Common adverse events associated with clofazimine are brown-orange or purple-red discolouration of skin, conjunctiva, cornea and body fluids; dry skin, pruritus, rash, ichthyosis and xerosis; gastrointestinal intolerance; and photosensitivity. Patients should be well informed from the outset of the reversible skin colour changes that occur in most patients. The orange-brown skin changes are reversible within a few months (sometimes more) of the drug being stopped and are not considered dangerous. These skin changes can be quite concerning to patients and reassurance is required. Clofazimine can be used during pregnancy or breastfeeding owing to limited data and to pigmentation of the infant if the drug is used during breastfeeding. Clofazimine is partially metabolized by the liver; hence, caution or adjustment of the dose is required for patients with severe hepatic insufficiency.
Cycloserine
Cycloserine is a bacteriostatic drug that inhibits cell wall synthesis, and it has no known cross-resistance to other TB medicines. Terizidone (composed of two molecules of cycloserine) may be used instead of cycloserine, and cycloserine and terizidone are considered interchangeable. Because of difficulties in interpreting DST (there is no reliable genotypic or phenotypic DST for cycloserine or terizidone), cycloserine or terizidone should only be considered when other criteria of likelihood of effectiveness are met; for example, any reliable evidence on population levels of drug resistance, and prior use of cycloserine or terizidone based on a reliable clinical history (Section 3.1). Patients should be well informed of the potential adverse events of cycloserine. A major drug adverse event is CNS toxicity, including inability to concentrate, depression, behaviour change (e.g. violence and aggressiveness, and suicidal ideation), frank psychosis, seizures and lethargy.
Cycloserine may exacerbate pre-existing neurologic or psychiatric conditions. Situations of stigma, extreme poverty and social vulnerability are not infrequent among MDR/RR-TB patients, and these also affect mental health. Depression and anxiety are also highly prevalent and can lead to a worse prognosis and loss to follow-up, especially in programmes without patient-centred systems. In these situations, management of cycloserine toxicity is critical to obtain good clinical outcomes and to avoid serious adverse events.
Group C
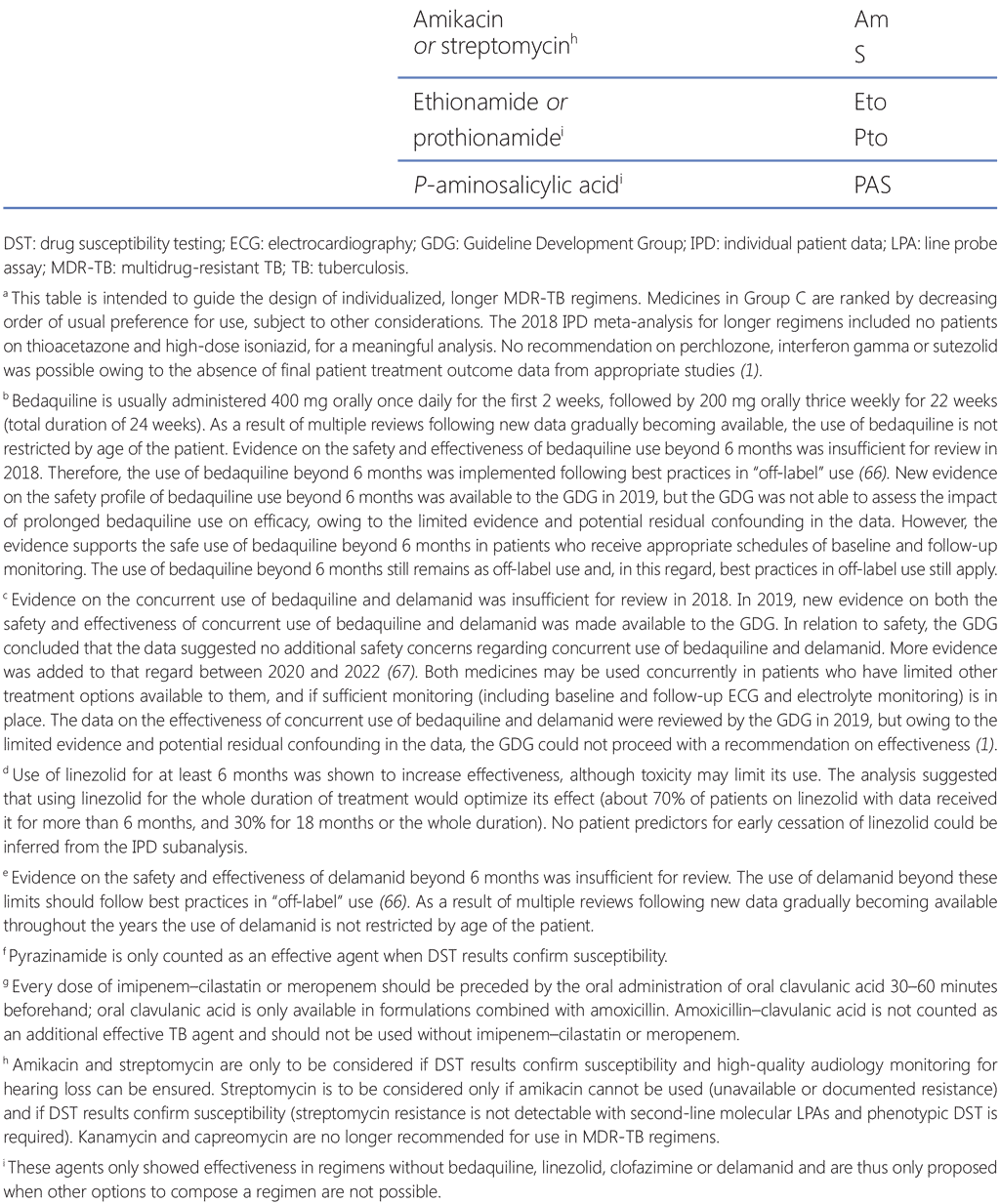
Group C comprises both TB and repurposed medicines that are positioned at a lower priority than the Group A and B agents, either because they are less effective (ethambutol, delamanid, pyrazinamide, ethionamide/prothionamide and p-aminosalicylic acid) or because they are more toxic and cumbersome to administer parenterally (imipenem–cilastatin, meropenem, amikacin and streptomycin). These drugs are usually included in a longer regimen if it cannot be composed with Group A and B agents alone.
Ethambutol
Ethambutol is a TB medicine that is used in the treatment of DS-TB and may be added to longer MDR-TB regimens. At recommended dosages, the safety profile of ethambutol is good. Owing to difficulties in interpreting its DST, ethambutol should only be considered when other criteria of likelihood of effectiveness are met (e.g. evidence on a population level of low prevalence of drug resistance in circulating MDR/RR-TB strains and no prior use of ethambutol based on a reliable clinical history).
Delamanid
Based on current evidence on its effectiveness and safety, delamanid is recommended for use as a Group C agent (1). Delamanid has a potent in vitro bactericidal activity and potential sterilizing activity; it is thought that nitroimidazooxazole derivatives generate reactive nitrogen species, including nitrogen oxide, which are responsible for cell poisoning in low metabolic states. There is no age restriction for use of delamanid and there are currently dispersible formulations that are preferred over crushing and dispersing adult tablets (47, 92). Delamanid is strongly bound to plasma proteins, resulting in low CNS penetration; however, studies in humans and animals with CNS TB suggest that delamanid could potentially play a beneficial role when other options are not available (93).
The recent data review for the WHO guidelines (1) suggested that there are no additional safety concerns for concurrent use of delamanid with bedaquiline. The combined QT effects, compared with bedaquiline or delamanid alone, were evaluated in an RCT of 75 patients (>3000 ECGs) (78). Studies undertaken between 2020 and 2022 had shown no increased toxicity with the use of delamanid beyond 6 months; they showed safety on the concomitant use of delamanid with bedaquiline, while increasing rates of survival of patients with restricted therapeutic options (67, 81).
Animal data show no evidence of teratogenicity. Although the case series of pregnant women on delamanid are small, all children had excellent birth outcomes, suggesting that pregnant women in need should not be denied access to delamanid. It can be considered for the treatment of DR-TB in pregnant women with restricted therapeutic options (50).
Pyrazinamide
Pyrazinamide has been routinely added to MDR-TB regimens except where there is a reasonable clinical contraindication for its use (e.g. hepatotoxicity), or other serious adverse event or drug resistance. However, reliable DST for pyrazinamide is not widely accessible; hence, this drug has often been used without DST or regardless of documented resistance. In the longer regimens, pyrazinamide is recommended for inclusion only when DST results confirm susceptibility (in such cases it is counted as one of the effective agents); in any other cases, if pyrazinamide is included in the regimen, it is not counted as one of the four effective drugs (94, 95). There are synergies between pyrazinamide and other medicines such as bedaquiline, through complex mechanisms of action targeting dormant bacteria.
Imipenem–cilastatin and meropenem
Imipenem–cilastatin (not used in patients aged <15 years) and meropenem are the only carbapenems that have an established role in MDR-TB regimens. They are administered intravenously – a major drawback that limits their more widespread use outside hospitals, especially in resource-constrained settings (96–100). Daily IV injections are not usually feasible unless there is a surgically fitted port (a port-a-cath) or a peripherally inserted central catheter connected to a major vein. Meropenem with clavulanate as part of regimens (usually also containing linezolid) for patients with MDR-TB and XDR-TB has been shown to improve culture conversion and survival (101–103). Clavulanic acid (as co-amoxyclav) is not a TB medicine but is an adjunct agent that is given orally each time a carbapenem dose is administered, about 30 minutes before the IV infusion. When included in a regimen, clavulanic acid is not counted as one of the TB agents, and it should not be used without the carbapenem.
Amikacin and streptomycin
Amikacin and streptomycin are the only two aminoglycoside antibiotics that can be used when options for composition of the treatment regimen are limited. Based on the evidence reviewed in 2018, amikacin and streptomycin were associated with lower rates of treatment failure or relapse and death when used in people with M. tuberculosis strains susceptible to amikacin or streptomycin. However, these drugs share the disadvantages and serious toxicities (i.e. ototoxicity and nephrotoxicity) of other injectable agents that are no longer recommended (i.e. kanamycin and capreomycin). Given the high frequency of streptomycin resistance in patients with MDR/RR-TB in many settings, and its extensive historical use as part of older first-line TB regimens in many countries, streptomycin is unlikely to have much use in MDR-TB regimens.
Ethionamide and prothionamide
In WHO guidance, ethionamide and protionamide are considered interchangeable. The WHO consolidated guidelines make a conditional recommendation against their use in longer MDR-TB regimens, reserving them for situations where multiple, more effective agents (e.g. bedaquiline, linezolid and clofazimine) cannot be used. Apart from the low bactericidal profile, use of ethionamide and prothionamide is limited because of poor gastrointestinal tolerance, which could be potentially linked to bad adherence. In pregnant women, these drugs are usually not recommended owing to poor tolerance, decrease in thyroid stimulating hormone (TSH) levels (which are fundamental for the development of the fetus) and concerns raised by effects in animal reproductive studies.
P-aminosalicylic acid
P-aminosalicylic acid (PAS) can be considered as the last resource for treatment of MDR/RR-TB. It is often poorly tolerated and has a modest bacteriostatic activity. The drug is recommended in the WHO consolidated guidelines only for use in the treatment of MDR/RR-TB patients on longer regimens if bedaquiline, linezolid, clofazimine or delamanid are not used, or if better options to compose a regimen are not possible. There is no indication of cross-resistance of p-aminosalicylic acid to other anti-TB drugs (1). Use of p-aminosalicylic acid is limited owing to poor gastrointestinal tolerance.
Other medicines
Some medicines previously recommended as potential components of MDR-TB longer treatment regimens do not feature in Groups A–C.
High-dose isoniazid
High-dose isoniazid is not included in Groups A–C given the rarity of its use in longer regimens for adults. It is considered a relatively safe medicine, as shown recently in experience with its use at the 10 mg/kg dose, where only 0.5% of 1006 patients in a multicentric observational study of the shorter MDR-TB regimen reported Grade 3 or 4 neurotoxicity (104). Other evidence suggests that high-dose isoniazid may also be useful in the longer MDR-TB regimens. First, in the systematic review and IPD meta-analysis commissioned by WHO in 2015 to describe treatment outcomes in children with MDR-TB (which included 975 children in 18 countries), the use of high-dose isoniazid was associated with treatment success among children with confirmed MDR-TB (adjusted odds ratio [aOR] 5.9, 95% confidence interval [CI]: 1.7–20.5, P=0.007) (105, 106). Second, in a randomized, double-blinded, placebo-controlled clinical trial among adults with MDR-TB, participants who received high-dose isoniazid (16–18 mg/kg) (added to kanamycin, levofloxacin, prothionamide, cycloserine and p-aminosalicylic acid) were significantly more likely to experience culture conversion at 6 months of treatment than those receiving placebo or standard-dose isoniazid (5 mg/kg) (73.8% versus 48.8% or 45.0%, respectively), with median time to culture conversion significantly reduced in the high-dose isoniazid arm (3.4 versus 6.6 or 6.4 months, respectively) (107). Third, a more recent early bactericidal activity study among patients with MDR-TB – in which the isoniazid resistance was mediated by isolated inhA mutations – demonstrated that doses of 10–15 mg/kg of isoniazid daily exhibited bactericidal activity similar to standard-dose isoniazid (5 mg/kg) given to patients with DS-TB (108). Strains with isolated katG or both katG and inhA mutations are unlikely to respond even to high-dose isoniazid, given the typically high isoniazid MICs in those strains. In the absence of information on isoniazid mutation patterns for an individual patient, knowledge of the prevalence of both mutations among locally circulating RR-TB strains (e.g. from DRS in the relevant epidemiological setting) may also inform decisions as to which treatment regimens would be most appropriate.
 Feedback
Feedback