Book traversal links for 5.2 Specific features and required biosafety measures
Similar to the moderate-risk laboratory, there are two levels of containment in a high-risk laboratory: the BSC (primary containment) and the laboratory itself (secondary containment).
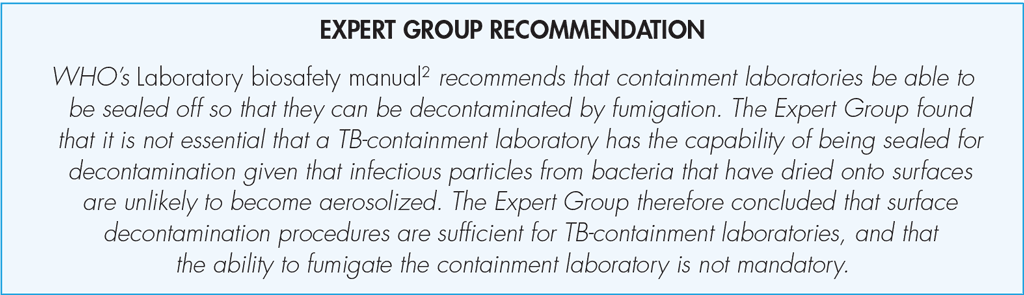
In TB laboratories classified as high risk, all procedures for handling viable M. tuberculosis cultures and aqueous suspensions of TB bacilli for identification, indirect DST and molecular assays must be conducted within a BSC in a TB-containment laboratory.
In addition to the safety elements required for a moderate-risk laboratory, a high-risk (or TB-containment) laboratory requires the following additional enhancements.
1. Laboratory design: Two sets of entry doors are essential to create an anteroom to the containment laboratory. This design provides a physical barrier between the containment section of the laboratory and the outer laboratory areas. It also permits a unidirectional flow of air into the laboratory The anteroom should have facilities for separating clean clothing from dirty clothing. Doors to the anteroom may be self-closing and interlocking so that only one door can be open at a time. A break-through panel may be provided for emergency exit. Air can flow into the TB-containment laboratory through the anteroom; and grills fitted with pre-filters can be placed in the lower panels of the anteroom’s doors to ensure that only clean air flows into the TB-containment laboratory.
A glass panel should be installed to give a view from the outer laboratory areas into the containment laboratory.
2. Personal protective equipment: Each facility must evaluate its risks and decide on the level of personal protection that is appropriate for staff.
Protective laboratory gowns must be worn. Gowns should have solid front panels and must be impermeable to liquids. Laboratory gowns should have long sleeves and an elasticized cuff (at least 30 mm long) and fasten at the back.
Gloves must be worn. Staff must always wash their hands before leaving the laboratory
The use of hair coverings, shoe covers or dedicated shoes are optional; they may be adopted as additional protective measures. However, any protective clothing used in the TB-containment laboratory must not be worn in the other areas of the laboratory.
Respiratory equipment provides additional protection during high-risk procedures – such as the manipulation of liquid cultures for identification and DST– that generate aerosols with high concentrations of infectious particles. Protective respiratory equipment should not be considered a substitute for a poorly functioning BSC or a BSC lacking certification. In all cases, good microbiological techniques are essential to minimize the risk of laboratory-acquired infections.
3. Decontamination and waste disposal: An autoclave must be available on site in the vicinity of the TB-containment laboratory to allow tubes and vials with cultures of TB bacilli to be sterilized prior to being removed for disposal. All other infectious waste needs to be removed from the TB-containment laboratory for proper disposal. It must be transported in sealed plastic bags or containers, following appropriate local regulations. Any materials that are reused must be decontaminated with a suitable disinfectant or autoclaved before being removed from the laboratory.
 Feedback
Feedback