Book traversal links for 5.1 Recommended TPT regimens
TPT falls broadly into three categories: (i) isoniazid monotherapy for 6 or 9 months (6H or 9H), (ii) rifamycin-based shorter treatment and (iii) Lfx for 6 months (6Lfx) for people exposed to MDR/ RR-TB. Isoniazid preventive treatment (IPT) for 6 months was the mainstay of TPT until recently, for both adults and children, HIV-positive and HIV-negative, and in high and low TB incidence countries. Several systematic reviews have consistently demonstrated the efficacy of IPT in preventing TB disease among people infected with M. tuberculosis. A systematic review of randomized control trials (RCTs) with people with HIV in 2009 showed that IPT reduces their overall risk for TB by 33% (RR 0.67; 95% CI 0.51 ; 0.87), and the preventive efficacy reached 64% for people with a positive TST (RR 0.36; 95% CI 0.22 ; 0.61) (56). This review also demonstrated that the efficacy of the 6-month regimen was not significantly different from that of 12-month daily isoniazid monotherapy (RR 0.58; 95% CI 0.3 ; 1.12). A systematic review of RCTs also showed a significantly greater reduction in TB incidence among participants given the 6-month regimen than in those given a placebo (odds ratio 0.65; 95% CI 0.50 ; 0.83) (57).
Evidence from clinical trials over the past two decades shows a similar preventive efficacy of a shorter rifamycin-based TPT regimen, in both HIV-positive and HIV-negative individuals, as monotherapy or in combination with isoniazid (56–60). The clear advantages of these regimens are better adherence due to the shorter duration and fewer adverse events. Use of the shorter rifamycin-based regimens is associated with at least 20% better treatment completion rate (82% vs 61%) (16). WHO has assessed and recommended several shorter rifamycin-based regimens as alternatives to 6H.
The external experts convened by WHO in GDGs to advise on treatment policies have assessed the evidence for the various TPT options, including the values and preferences of the beneficiaries and other considerations, such as regimen acceptability, feasibility, resource implications and likely impact on health equity. With these elements, the GDGs recommended various regimens with which the benefits are likely to outweigh potential harms of acquiring TB disease or drug toxicity. When choosing a regimen, health caregivers and the person taking the treatment should consider circumstances that would increase the likelihood of it being completed. The choice may also depend on the availability of resources, fixed-dose combinations (FDCs), child-friendly formulations, concomitant medication (such as antiretroviral drugs, ARVs), opioid substitution therapy, oral contraception), as well as its acceptability to recipients in the country context. Table 3 summarizes the characteristics of all currently recommended TPT options. The GDGs recommend that cost considerations not be a barrier to the provision of advantageous interventions such as dispersible drug formulations and tests of TB infection.
The 2020 guidelines broadened the applicability of a number of previous recommendations on testing for TB infection and TPT treatment regimen options from low-TB burden settings to any TB burden setting, on the condition that the country or treatment site has the capacity to rule out TB disease reliably before starting TPT, resources are available to implement TPT properly and measures are in place to limit the risks of TB infection and reinfection.
Since 2011, WHO has recommended IPT and other alternative regimens for people with HIV and other at-risk populations. In 2018, WHO recommended weekly rifapentine plus isoniazid for 3 months (3HP) and 3 months of daily isoniazid plus rifampicin (3HR) as options for TPT. In the 2020 update of the WHO guidance, two new regimens were added: (i) daily rifapentine plus isoniazid for 1 month (1HP), and (ii) daily rifampicin monotherapy for 4 months (4R). These, with 6H, are proposed as equivalent alternatives to TPT for individuals exposed to drug-susceptible TB in all settings. In 2024, a recommendation was added for use of 6Lfx in individuals exposed to MDR/RR-TB.
Table 3. Key characteristics of currently recommended TPT options
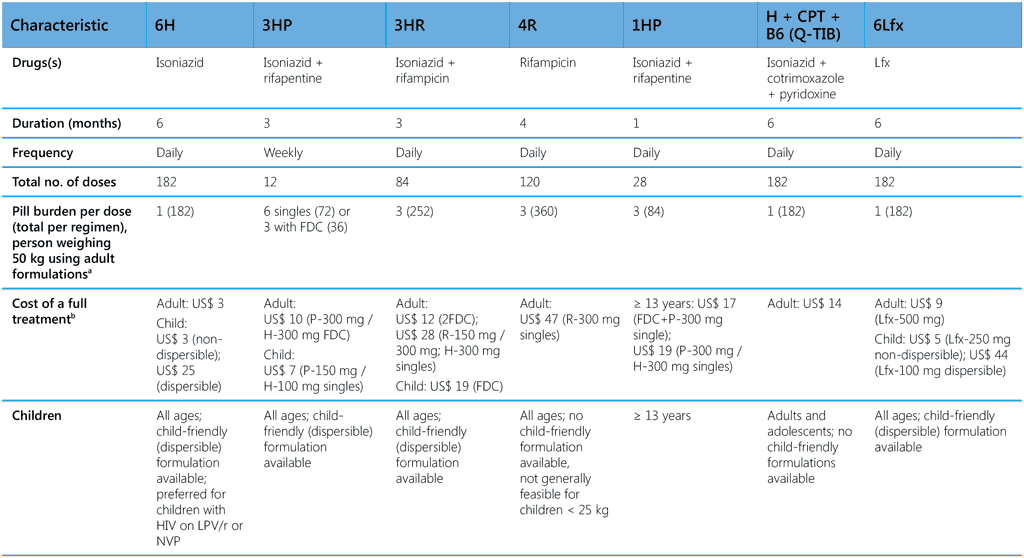
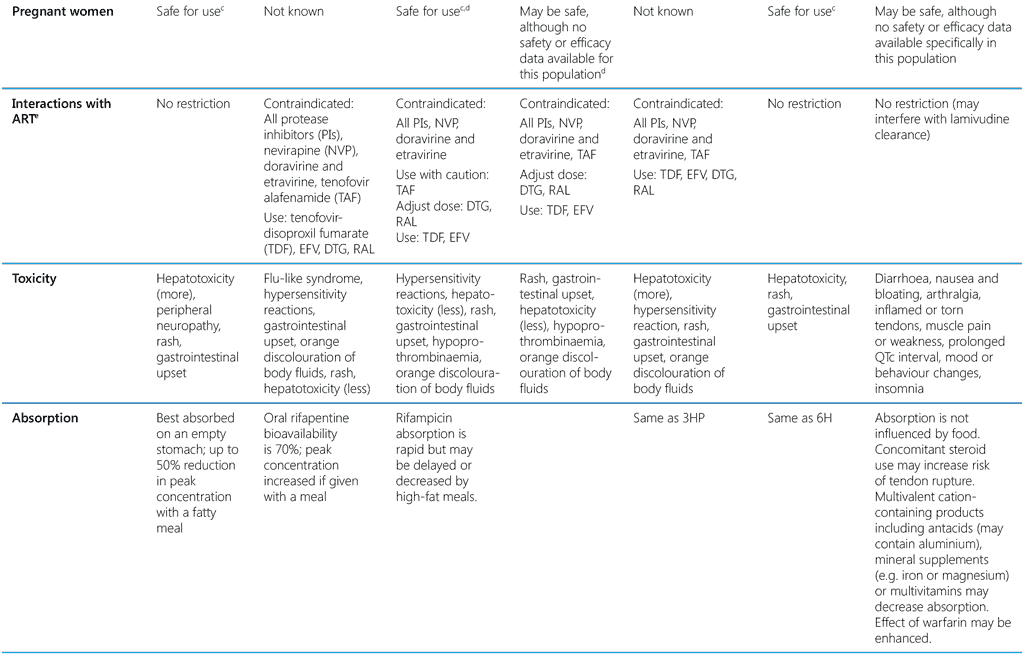
1HP, 1 month of daily rifapentine plus isoniazid; 3HP, 3 months of weekly rifapentine plus isoniazid; 3HR, 3 months of daily rifampicin plus isoniazid; 4R, 4 months of daily rifampicin monotherapy; 6H, 6 months of daily isoniazid monotherapy; 6Lfx, 6 months of daily levofloxacin monotherapy; B6, pyridoxine; CPT, cotrimoxazole; DTG, dolutegravir; EFV, efavirenz; FDC, fixed-dose combination; H, isoniazid; LPV/r, lopinavir–ritonavir; NVP, nevirapine; P, rifapentine; PI, protease inhibitor; H + CPT + B6 (Q-TIB), isoniazid- cotrimoxazole-pyridoxine combination; R, rifampicin; RAL, raltegravir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate
a H-300 mg; P-300 mg; H-300 mg/P-300 mg FDC; R-150 mg/H-75 mg FDC; R-300 mg/150 mg; Lfx-500 mg
b Approximate pricing for child (~12 kg) or average adult provided by the Stop TB Partnership Global Drug Facility (May 2024)
c One RCT has shown increased risk of poor birth outcomes for mothers taking isoniazid during pregnancy (69); however, other studies have shown benefits of IPT (13).
d Bleeding attributed to hypoprothrombinaemia has been reported in infants and mothers after use of rifampicin in late pregnancy. Vitamin K is recommended for both mother and infant postpartum if rifampicin is used in the last few weeks of pregnancy.
e In women receiving rifamycin-based TPT and oral contraceptives, consider additional barrier contraception methods to prevent pregnancy.
5.1.1 Isoniazid monotherapy
6H or 9H has been used most often for TPT worldwide. Isoniazid is, however, increasingly being replaced by rifamycin regimens, which are becoming more affordable and feasible and with more evidence on their efficacy and safety in different populations. It is likely that 6H or 9H will continue to be an important choice for TPT, particularly in situations in which rifamycin-based regimens cannot be used. In such situations, national programmes may consider use of a triple-pill combination of isoniazid, cotrimoxazole and vitamin B6 for people with HIV, which is available at a discounted price through the Stop TB Partnership’s Global Drug Facility, instead of an isoniazid-only regimen (61). Isoniazid may remain the preferred regimen for HIV-infected children on a protease inhibitor-based regimen (lopinavir–ritonavir), nevirapine or integrase inhibitors (dolutegravir) because of potential drug–drug interactions, until more evidence becomes available. Isoniazid monotherapy should also protect contacts of TB patients with laboratory confirmed isoniazid-susceptible, rifampicin-resistant disease (mono RR-TB).
In the second edition of the WHO TPT guidelines in 2024 (13), the conditional recommendation for ≥ 36 months of daily IPT for adults and adolescents with HIV in settings with high TB transmission has been withdrawn. The recommendation was based on low-certainty evidence from a systematic review and meta-analysis of three RCTs (62). In two of the studies reviewed, ART was not used, and, in the third, ART coverage was low at baseline but increased during the period of observation. Since the first release of the recommendation in 2011, country uptake has been poor, while access to ART has increased substantially worldwide. Shorter TPT options are preferred to IPT.
5.1.2 Three months of weekly rifapentine plus isoniazid and one month of daily rifapentine plus isoniazid
National programmes may consider either of these two rifapentine-containing regimens. The efficacy of both has been shown to be similar to that of isoniazid for TB prevention, but there is currently no direct evidence of efficacy from a comparison of 1HP and 3HP, although results from ongoing comparative studies are expected shortly.
WHO first recommended use of 3HP in 2018. At the time, however, the cost of rifapentine was a major barrier to uptake of 3HP. Large-scale interventions by global partners to shape the market for rifapentine resulted in introduction of generic products, which reduced the price of the 3HP regimen significantly, and it is now one of the preferred shorter TPT options throughout the world. It is recommended for use in people of all ages, including children < 2 years. Table 4 provides the recommended weight-band dosage down to 3 kg. (See also section 5.2.) The availability of child-friendly rifapentine and isoniazid formulations and an adult FDC make programmatic use of 3HP even more feasible. Further data on its use in pregnancy and with dolutegravir are expected shortly.
WHO first recommended use of 1HP in 2020. This regimen can be used in people aged ≥ 13 years, which was the age limit for the study population in the single RCT of the regimen for which results have been published to date (63–66). Further data are expected that could help to establish the appropriate dose of daily rifapentine for children < 13 years (67). The 1HP regimen may be used when a shorter duration is preferred, even if the total number of doses increases from 12 in 3HP to 28. The latter duration is used for prisoners incarcerated for a short term, patients awaiting anti-TNF treatment or preparing for a transplant, and people who must complete TPT before migration.
Table 4. Drug dosage schedule for TPT regimens according to body weight band
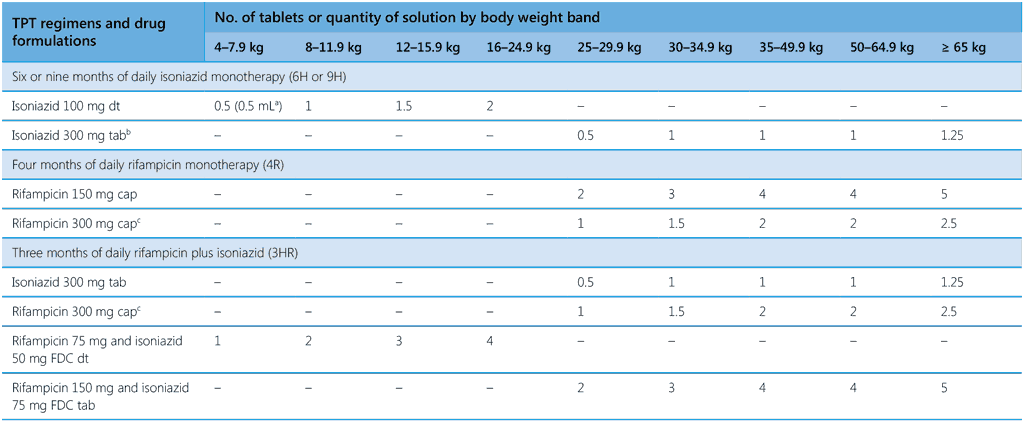
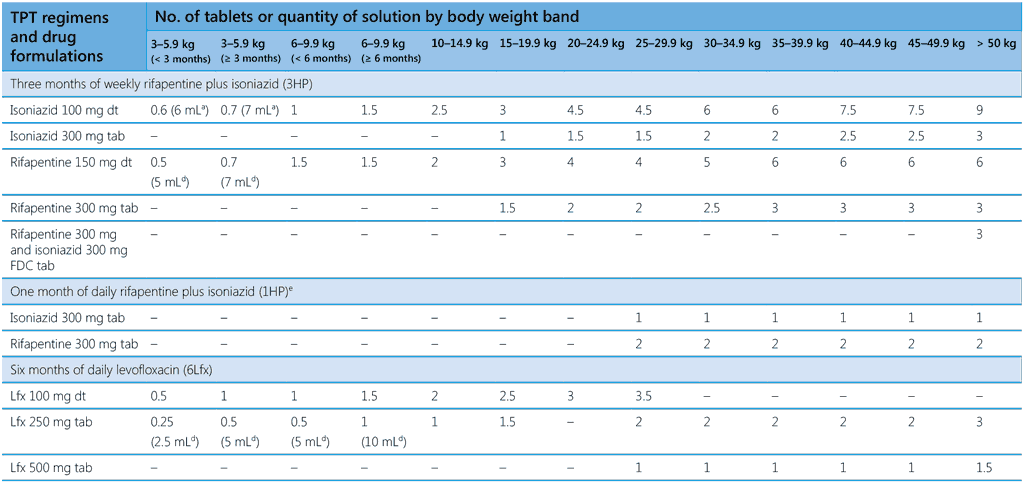
1HP, 1 month of daily rifapentine plus isoniazid; 3HP, 3 months of weekly rifapentine plus isoniazid; 3HR, 3 months of daily rifampicin plus isoniazid; 4R, 4 months of daily rifampicin monotherapy; 6H or 9H, 6 or 9 months of daily isoniazid monotherapy; 6Lfx, 6 months of daily levofloxacin monotherapy; dt, dispersible tablet; FDC, fixed-dose combination; kg, kilogramme; mg, milligramme; mL, milliliter; tab, tablet
please note different weight bands are used in the two parts of this table; a process of weight-band harmonization is ongoing.
a Solution with a concentration of 10 mg/mL (one 100 mg isoniazid dispersible tablet in 10 mL water)
b A triple pill combination of isoniazid 300 mg + pyridoxine 25 mg + sulfamethoxazole 800 mg + trimethoprim 160 mg (scored) can be used for people with HIV.
c A quantity of 0.5 can be achieved by adding a 150-mg capsule of rifampicin.
d Solution with a concentration of 15 mg/mL (one 150-mg rifapentine dispersible tablet in 10 mL water)
e For individuals aged ≥ 13 years
5.1.3 Three months of daily rifampicin plus isoniazid
Children < 5 years are particularly vulnerable because of increased risks of progression to TB disease and of developing severe forms of TB, such as TB meningitis and disseminated TB. In addition, it is difficult to confirm TB disease, given its paucibacillary nature. Therefore, averting paediatric TB by delivering preventive treatment is strategically important. For TPT among children, the 3HR regimen is better tolerated and more child-friendly than isoniazid, as dispersible FDC formulations are available. Until access to the child-friendly rifapentine formulations (which became available only in November 2023) is enhanced, national programmes could consider 3HR as an option for TB prevention among children of all ages. Those weighing < 25 kg (including children < 2 years) may receive the RH formulation used for the continuation phase of TB treatment (R/H, 75/50 mg), while children weighing > 25 kg may receive either 3HP or 3HR as adult FDCs of RH. Child-friendly FDCs of RH have the added benefit that they are already in the national supply chain for TB treatment of children weighing < 25 kg. In adults, however, the risk of hepatotoxicity with use of 3HR is expected to be as high as with 6H or 9H, and 3HP may be the preferred option.
In the medium to long term, 3HP (or 1HP) may become the preferred regimen for all ages. This document provides updated information on the dosage of 3HP for children, including those < 2 years. Dispersible formulations of rifapentine (150 mg scored) and isoniazid (100 mg) are increasingly becoming available. The shorter duration of treatment with 3HP and the higher rates of treatment completion will probably make it more cost-effective in the long term.
5.1.4 Four months of daily rifampicin monotherapy
Rifampicin has a long history of use in TB treatment, and national procurement systems have experience in acquiring it, usually with other TB medicines in FDC tablets. Rifampicin has a much better safety profile than isoniazid, and its cost is lower than that of rifapentine. WHO recommends daily rifampicin for 4 months as one TPT option, which may also be given to contacts of people with confirmed isoniazid-resistant, rifampicin-susceptible TB disease. Some of the main challenges with 4R, however, are the perception that rifampicin should be protected for use as first-line TB medicine and concern that its use in TPT may increase rifampicin resistance in the community or promote misuse of the agent as monotherapy for TB disease. There is, however, no evidence of a significant increase in rifampicin resistance due to scaling up of TPT services. Other possible challenges are drug–drug interactions with ARVs (see section 6), the current lack of child-friendly formulations and the limited supply of single-dose formulations due to the widespread availability of FDCs of first-line TB treatment.
5.1.5 Levofloxacin and other TPT regimens for drug-resistant TB
The second edition of the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment, published in 2024 (13), contains an updated recommendation on TPT for individuals exposed to MDR/RR-TB. WHO now recommends 6Lfx as a TPT option for people exposed to MDR/ RR-TB in all settings, subject to specific conditions. Lfx is the preferred choice of fluoroquinolone for TPT, and the recommendation is based on evidence from two randomized controlled trials with this drug – TB CHAMP and V-QUIN, a systematic review of studies on use of TPT for MDR/RR-TB and studies on the programmatic feasibility and acceptability of 6Lfx (13).
Confirmation of infection by TST, TBST or IGRA before starting TPT for MDR/RR-TB is not required for child contacts and people with HIV or other immunocompromising conditions. In other populations, this is desirable but not mandatory, and the lack of availability of testing should not be a barrier to providing TPT to individuals at risk of MDR/RR-TB. If Lfx is used for TPT of MDR-TB, TB disease must be carefully excluded to limit the risk of emergence of resistance to fluoroquinolones – key components of second-line TB treatment regimens – should the person subsequently require treatment for MDR-TB disease. TB disease should be ruled out by an appropriate clinical evaluation or according to national guidelines. Provision of TPT with Lfx should also account for factors such as age, risk of toxicity or interaction, co-morbidity, drug susceptibility of the strain of the most likely source case, background resistance to fluoroquinolones in MDR/RR-TB strains, availability and the individual’s preferences.
While there are no comparable data to support use of alternatives, moxifloxacin can be used if Lfx is not available, and the dosage proposed for treatment of MDR/RR-TB can be used (66). Drug-susceptibility testing of the strain from the presumed source patient would be important additional information, especially in situations where fluoroquinolone resistance is known to be high. If the strain of the source patient shows resistance to these medicines, other TB drugs (e.g. ethionamide, ethambutol) can be used for TPT, with the best available information on the drug susceptibility profile of the presumed strain. In this case, the certainty of the effectiveness of TPT is much lower than with Lfx. Results from the PHOENIx trial, in which 26 weeks of delamanid are compared with isoniazid for household contacts (all ages) of MDR-TB patients in 11 countries are expected in mid-2025 (67).
Contacts of individuals with rifampicin-resistant TB may be treated similarly to those with MDR-TB; if susceptibility to isoniazid is confirmed in the presumed source patient, contacts may be given IPT.
5.2 Recommended dosage of TPT medication
Table 4 presents an updated dosing scheme for all recommended TPT regimens by standardized body weight-bands. In 2024, the Technical Advisory Group on dosing of TB medicines for adults and children (70) reviewed questions relating to the dosage of TPT regimens. They considered published evidence and the results of pharmacokinetics modelling and simulation in studies of 6Lfx and 3HP. (See details in Web Annex A.) Dosing of smaller children is adjusted for differences in the rate at which drugs are metabolized. For infants and young children, age cut-offs are given in the first two weight bands for 6Lfx and 3HP. It is important to note that infants in these two weight bands may be malnourished, a condition that warrants particular care to exclude TB disease before TPT is considered. A specialist should be consulted for dosing of TPT regimens for infants who weigh ≤ 3 kg.
Weight-band dosing facilitates administration of medication by front-line staff. The doses shown in Table 4 also take into account the most common drug formulations available on the market. When more than one formulation of the same medicine is commonly available, doses are shown for each option. For three regimens, the weight bands used adhere to the harmonized approach recommended by WHO for dosing schedules. (The weight bands in the first part of Table 4 do not adhere to the harmonized approach.) The recommended dosing is designed to avoid splitting of non-dispersible tablets into fractions smaller than one half. When administering treatment, factors should be considered that may increase the risk of drug toxicity, such as malnutrition, co-morbidity and drug–drug interactions. These factors should also be reflected in job aids for health-care providers. In settings in which many people are known to be slow acetylators (mutation in the NAT2 genotype leading to persistence of isoniazid),1 national programmes may consider pyridoxine supplementation for all individuals receiving isoniazid-containing TPT (71). (See also section 5.2.2.) More empirical data should be made available for settings with different distributions of slow and fast isoniazid acetylators in order to develop better guidance on the selection of doses that ensures therapeutic exposure to isoniazid-based TPT without reaching toxic levels.
5.2.1 Availability of appropriate formulations
Rifampicin: The most widespread use of rifampicin is in rifampicin-containing FDCs to treat drug-susceptible TB, and single-dose rifampicin is less commonly procured. It is therefore likely to be available in only limited quantities to national programmes. Additionally, child-friendly dispersible formulations of single-dose rifampicin are not currently available. The development of a 100-mg scored, dispersible rifampicin tablet may be useful for rifampicin-containing TPT in children. If the demand for the 4R regimen increases, programmes will have to increase their orders for single-dose rifampicin capsules. In this case, use of rifampicin should be regulated and limited for use as part of the TPT regimen, to avoid it being diverted for use as a broad-spectrum antibiotic. The supplies of 4R to peripheral centres (primary-care facilities, HIV programmes) should be accompanied by specific guidance on use of rifampicin.
Isoniazid plus rifampicin FDC: Child-friendly dispersible FDC of HR are available and are already used in many countries for treatment of TB disease in children. The same formulations can be used for TPT. Child-friendly FDCs of HR should be preferred over single-dose formulations, to reduce the pill burden. Similarly, FDCs used for treatment of adult TB disease may be used for TPT among adults. The dispersible formulation is currently more expensive than solid formulations.
Isoniazid plus rifapentine (weekly or daily): Regimens based on these two drugs are now more feasible for use by people of all ages, in view of the formulations available, such as child-friendly tablets of isoniazid and rifapentine that are dispersible in water and have a fruity flavour. While they are currently more expensive than solid formulations, they allow more precise, reliable dosing of children than crushing of adult tablets (72). 3HP or 1HP can be given in different combinations of single-dose isoniazid (100-mg scored dispersible paediatric and 100-mg or 300-mg solid formulations), single-dose rifapentine (150-mg scored dispersible paediatric and 300-mg solid formulation) or as an FDC of isoniazid 300 mg and rifapentine 300 mg (73). The 300-mg rifapentine formulation reduces the pill burden of both 3HP and 1HP for adults. The FDC will further reduce the weekly pill burden for 3HP in adults weighing ≥ 50 kg from nine to three pills and the daily pill burden for 1HP to just one FDC plus one rifapentine 300-mg capsule. The child-friendly rifapentine and isoniazid formulations will increase the flexibility and ease of administration of 3HP to young children, including those aged < 2 years. Functional scoring of rifapentine tablets allows use of 75-mg dose increments for different weight bands. The generic product of the 150-mg rifapentine scored dispersible tablet that is on the market has been approved by the Global Fund Expert Review Panel and can be ordered from the Stop TB Partnership Global Drug Facility (74). The tablet has a raspberry–mint flavour when dispersed in water. A small volume of about 10 mL is required to disperse a tablet (it might have to be increased if several dispersible tablets are taken at once) (72). It has a shelf-life of about 24 months. In addition, WHO has prequalified a 100-mg scored dispersible tablet formulation of isoniazid, which is fruit flavoured for greater palatability. The FDC containing 300 mg isoniazid and 300 mg rifapentine is also available from generic manufacturers, and access to child-friendly formulations is being facilitated by the Unitaid-funded IMPAACT4TB Consortium (74). The Treatment Action Group is campaigning for access to rifapentine-containing regimens through the 1/4/6x24 campaign (75).
Isoniazid + cotrimoxazole + pyridoxine combination: This combination is available at a discounted price through the Stop TB Partnership Global Drug Facility and the Global Fund Pooled Procurement Mechanism. These combination pills may be considered alternatives for people with HIV when shorter rifamycin-containing regimens are not available or drug–drug interactions occur. They are single-scored tablets and cannot be used for children aged < 5 years.
Levofloxacin is available as 100-mg scored dispersible paediatric tablets and as 250-, 500- or 750-mg solid formulation tablets. The dispersible formulation is currently more expensive than the solid formulations.
5.2.2 Role of pyridoxine and its availability
Pyridoxine (vitamin B6) in the diet is converted into coenzymes that play an essential role in the metabolism of protein, carbohydrates, fatty acids and several other substances, including brain amines. Isoniazid apparently competitively inhibits the action of pyridoxine in these metabolic functions (76) and is associated with neurotoxicity and peripheral neuropathy
Individuals at risk for isoniazid-induced peripheral neuropathy include those with malnutrition, chronic alcohol dependence, HIV infection, renal failure and diabetes, as well as pregnant and breastfeeding women and infants who are exclusively breastfed by mothers on isoniazid. The earliest symptom of isoniazid-induced neurotoxicity is usually paraesthesia, followed by pricking pain and a burning sensation in the feet and later in the hands (symmetrical numbness and tingling). If untreated, the symptoms worsen and cause distress. These symptoms are easily recognized and are usually easily reversed upon withdrawal of isoniazid and institution of pyridoxine therapy.
The incidence of peripheral neuropathy is closely correlated with the dose of isoniazid used. Studies conducted mainly in the 1950s (77–80) reported that, while > 40% of people receiving high-dose isoniazid (16–24 mg/kg per day) developed signs and symptoms of peripheral neuropathy, only 2% of those receiving a standard dose of 4–6 mg/kg/day did so. The incidence of neuropathy was, however, observed to be higher at a standard isoniazid dosage in malnourished patients (up to 20%) (78) and among slow acetylators of isoniazid, reaching 20%. (See also section 5.2) Signs of toxicity tend to appear later among those taking standard doses of isoniazid.
Pyridoxine supplementation may be required to prevent or treat isoniazid-associated neurotoxicity. While a standard dose of isoniazid is used in IPT, the weekly dose of isoniazid in 3HP is higher. Routine administration of pyridoxine supplements to otherwise healthy individuals on a standard dose of isoniazid is not usually required (77). An adequate human diet containing 1–2 mg of vitamin B6 compounds daily may protect against isoniazid toxicity. Good dietary sources of vitamin B6 include carrots, spinach, peas, potatoes, milk, cheese, eggs, fish, meat and fortified flour. Concurrent administration of pyridoxine with isoniazid protects individuals at risk from the development of peripheral neuropathy; the recommended dose is 10–25 mg/day. For established isoniazid-induced peripheral neuropathy, pyridoxine should be given at a higher dose of 50–75 mg daily and even up to 100–200 mg per day (79). It is important to maintain pyridoxine supplementation at the right dose, as higher levels may interfere with the antibacterial activity of isoniazid. Moreover, excessively high doses of pyridoxine (≥ 2000 mg/day) have been reported to cause toxicity, including peripheral neuropathy (80–84).
The pyridoxine formulation currently available in the Global Fund’s Pooled Procurement Mechanism and the Global Drug Facility products list are 10-mg film-coated tablets, 50-mg uncoated tablets and 100-mg film-coated tablets (61). The latter formulations are suitable primarily for therapeutic use and are difficult to fraction into the doses recommended for prophylactic supplementation. National programmes may consider local procurement of a quality-assured product of lower-dose pyridoxine (10–25 mg) for use in high-risk individuals or, alternatively, procure vitamin B complex. Use of isoniazid– B6–cotrimoxazole combination tablets may be considered for people with HIV. Programmes are nonetheless advised to stock higher-dose pyridoxine for treatment of peripheral neuropathy. It is important that programmes do not delay initiation of TPT if procuration of pyridoxine is difficult.
5.3 Provision of TPT for special populations
5.3.1 Pregnancy and postpartum period
Pregnancy increases the risk of progression from TB infection to disease and the risk of poor maternal and fetal outcomes should TB disease occur. Women with HIV are at higher risk for TB during pregnancy and the postpartum period, which can have severe consequences for both the mother and the infant (85,86). Pregnancy should therefore not disqualify women from receiving TPT if they are eligible for it, regardless of their HIV status. Isoniazid and rifampicin, the medicines commonly used in preventive treatment, are considered safe for use in pregnancy (classified as Pregnancy Category C by the US Food and Drug Administration) (87,88).
Preventive treatment with isoniazid and/or rifampicin can be safely given to breastfeeding women (89). One trial showed an increased risk of adverse pregnancy outcomes with IPT (69); however, no other studies have shown an association of IPT with fetal or neonatal death, prematurity, low birth weight or congenital anomaly. Similarly, no statistically significant risks for maternal hepatotoxicity, grade 3 or 4 events or death were reported. A study published in 2023 showed no difference in infant acquisition of TB between mothers with HIV who received IPT during pregnancy or post partum (90). Therefore, systematic deferral of TPT to the post-partum period is not necessary. When indicated, IPT should be started during the antenatal and postnatal periods, with due care. The triple-pill combination of isoniazid + cotrimoxazole + B6 may be used for TPT among pregnant and breastfeeding women with HIV. Rifampicin is generally considered safe for use during pregnancy, and no dose adjustment is necessary, although no data are available on its safety or efficacy in pregnant and post-partum women on 4R as TPT (91).
Few data were available on the efficacy and safety of rifapentine in pregnancy. Programmatic use of 1HP and 3HP in pregnancy is thus cautioned until more data become available. One clinical trial (WHIP3TB) of the outcomes of women who initiated 3HP and became pregnant showed a similar frequency of spontaneous abortion and adverse pregnancy outcomes (when analysed as a composite outcome) in the exposed and unexposed groups (92). A study of the pharmacokinetics and safety of 3HP in pregnant women showed that dose adjustment would not be required to achieve therapeutic levels in pregnancy. Although rifapentine clearance was higher among women with HIV, their exposure was considered sufficient for TB prevention (93).
The risks and benefits of TPT with Lfx in pregnancy should be assessed, and pregnant women should be enabled to make an informed choice about whether to take TPT or to defer TPT to the end of pregnancy. The advice given should be adapted to the circumstances (e.g. use in the first trimester or later). MDR/RR-TB in pregnancy is a serious condition, and some of the drugs used to treat MDR-TB may be toxic to the fetus. Observations from studies in animals exposed to Lfx have limited its use in pregnancy; however, one meta-analysis of observational studies of 2800 pregnant women given fluoroquinolones for any indication (e.g. urinary tract infection) found no difference in the incidence of birth defects, spontaneous abortion or prematurity from that in unexposed pregnant women (94). Lfx concentrations in breastmilk appear to be far lower than the infant dose and would not be expected to cause adverse effects in breastfed infants (95); therefore, Lfx should not be suspended during breastfeeding. While the effects of fluoroquinolones on bone and cartilage in animals have not been reproduced in humans, data and infant follow-up are limited. Recent alerts have raised safety concerns associated with prolonged use of fluoroquinolones in humans (96–98).
Routine liver function testing is not indicated when TPT is given during pregnancy, unless other hazards are present. Pyridoxine (vitamin B6) supplementation should be given routinely to all pregnant and breastfeeding women on isoniazid-containing TPT. Pyridoxine should be given to infants who are taking isoniazid or whose breastfeeding mothers are taking isoniazid.
5.3.2 Infants born to mothers with TB disease
- Assess the newborn. If the newborn is unwell, refer him or her to a specialist or a paediatrician. It is important that the mother receives effective TB treatment so that she is no longer infectious. Ensure that infection control measures are in place in the nursery, especially in an inpatient facility for the care of preterm or small-at-birth infants.
- If the newborn is well (with no signs or symptoms suggestive of TB), provide TPT and delay BCG vaccination until two weeks after TPT is complete. Administer pyridoxine at 5–10 mg/day. Exclusion of TB disease is particularly critical in infants who are malnourished before starting TPT.
- Infants born to mothers who are HIV-positive and are on nevirapine should also receive IPT. Rifamycinbased TPT cannot be given with nevirapine prophylaxis, as rifampicin and rifapentine decrease nevirapine levels and may thus increase the risk of mother-to-child transmission of HIV (99).
- At the end of TPT, perform a TST or IGRA test. If the test for TB infection is negative or not available, give BCG (unless the infant is HIV-positive).
- If the mother is taking anti-TB drugs, she can safely continue to breastfeed. The mother and infant should stay together, and the infant may be breastfed while on TPT. Infants being breastfed by a mother who is on TPT should receive pyridoxine for the duration of the mother’s treatment.
5.3.3 Women taking oral or hormonal contraceptives
Rifampicin and rifapentine interact with oral and hormonal contraceptive medications, with a potential risk of decreased contraceptive efficacy. Women who are taking oral contraceptives while on rifampicin or rifapentine should either:
- change the oral contraceptive pill and use an alternative, such as depot medroxyprogesterone acetate every eighth week (100) or higher-dose oestrogen (50 μg) in consultation with a clinician; or
- use another form of contraception, a barrier contraceptive or an intrauterine device.
In women with hormonal contraceptive implants, the interval for replacing the implants might have to be shortened from 12 to 8 weeks (100).
5.3.4 People with hepatitis or liver disease
Isoniazid, rifampicin and rifapentine are associated with liver dysfunction. TPT should be initiated with caution among individuals whose baseline liver transaminase values are found to be more than three times the upper limit of normal. TPT should not be given to individuals with end-stage liver disease. IPT is, however, well tolerated by individuals with chronic hepatitis B or hepatitis C infections (101,102). In people with acute hepatitis due to infection or another cause, TPT should be deferred until the condition has resolved. Rifampicin and rifapentine can decrease the concentration of direct-acting ARV used to treat hepatitis C infection to subtherapeutic levels, and their use together is therefore not recommended (103,104). People with hepatitis C should consult their health-care providers and start rifamycin-based TPT either before or after completing treatment for hepatitis C.
5.3.5 People with renal failure
Isoniazid, rifampicin and rifapentine are eliminated by biliary excretion and can therefore be given in standard dosages to patients with renal failure. Patients with severe renal failure should receive isoniazid with pyridoxine to prevent peripheral neuropathy.
5.3.6 People with HIV
A key challenge in TPT with rifamycin-based regimens for people with HIV is drug–drug interaction between rifamycin and antiretroviral drugs (105). No dose adjustment is required when rifapentine or rifampicin is co-administered with efavirenz. The dose of dolutegravir should be increased to 50 mg twice daily when given with rifampicin; no dose adjustments are necessary when rifapentine is used. Rifampicin or rifapentine TPT regimens should not be co-administered with protease inhibitors or nevirapine (for details, see section 6.3).
5.3.7 People who use drugs
The prevalence of TB infection and incidence of TB disease is higher among people who use drugs (106). IPT is safe for these people, although careful monitoring for liver toxicity is important. No systematic study of use of rifapentine by people who use drugs has been conducted; however, rifampicin is known to reduce exposure to opioid substitution therapy such as methadone and buprenorphine (107). In some people, this results in opiate withdrawal. For this reason, people taking 3HP, 3HR or 4R with opiate substitution therapy should be closely monitored for signs of opiate withdrawal and other adverse events. Increasing the dose of methadone or buprenorphine for people taking rifamycins can lessen the risk of withdrawal. Drug use should never be considered a blanket reason for denying someone TPT. It is the responsibility of health-care providers to proactively manage drug–drug interactions for people who use drugs safely (108).
5.3.8 TB among older adults
Many countries are experiencing demographic shifts as average life expectancy increases, with larger numbers and proportions of older adults in their populations (109). TB remains one of the foremost infectious causes of disease and death among ageing adults. About 12% of all notified TB patients worldwide today are over 64 years of age, although the proportion is much higher in some countries (e.g. 30% in China, 70% in Japan). In settings with a lower TB burden and limited community transmission, the TB epidemic is driven by TB reactivation in older adults as their immunity wanes; however, TB reactivation in older adults is also relevant in high-burden settings and contributes to ongoing community transmission.
WHO recommends screening, testing for TB infection and provision of TPT to people exposed to TB, regardless of age, and to people with clinical conditions due to immune suppression. These recommendations must also be implemented for older people. National programmes should: promote the acquisition of local evidence on gaps in preventive care for older adults; invest in enhancing access to testing for TB infection and to shorter rifamycin-containing TPT; provide guidance on risks and benefits before TPT is initiated in older adults; and monitor adverse events in people on TPT. Routine TB screening and care of people living in homes for the elderly, provision of age-friendly infrastructure and services, awareness of atypical TB features, integration of TB and noncommunicable disease services, and person-centred approaches to treatment support could enhance access to TPT and improve TB management among older adults.
5.4 Duration of protection
The durability of protection from TB is a function of both the potency of the TPT regimen to sterilize TB infection and the risk of re-infection after treatment. TB infection that is not adequately treated because of an inadequately potent regimen or poor adherence to treatment may result in reactivation of TB infection, leading to TB disease.
People with HIV are at high risk of reactivation of TB infection and of progressing to TB disease when infected. Studies conducted before ART found an escalating risk for TB after a course of TPT in high-TB burden countries, while more lasting protection was observed in countries with a low or medium burden of TB in terms of reduced mortality and incident TB. Recent trials conducted since widescale access to ART, however, suggest that the protection offered by TPT, even in high TB burden settings, can last as long as in countries with a low or medium TB burden.
- In Côte d’Ivoire, where TB incidence was reported in 2017 as 159 per 100 000 people, 6 months of IPT had a strongly protective effect against mortality among HIV-infected people who had started ART, even in those with a high CD4 cell count, and the protective effect lasted for up to 6 years (21).
- In Brazil, which has a medium prevalence of TB, IPT significantly reduced the risk for TB of HIVinfected patients with a positive TST. A 6-month course of isoniazid reduced the TB risk for > 7 years. In studies in high-burden settings in Africa, however, the TB incidence increased immediately after IPT (110,111).
- Recent studies in Indonesia and Myanmar, which are high TB burden countries, reaffirm the durability of protection after 6 months of IPT among people with HIV. In Indonesia, the protective benefit lasted > 5 years (112). In Myanmar, completing a course of IPT significantly reduced the risk of TB disease and death for as long as 8 years (113).
- In the BRIEF-TB trial, in which 97% of participants were in high TB burden countries, the TB incidence after a complete course of TPT with either 1-month isoniazid and rifapentine or a 9-month isoniazid regimen remained stable throughout the 3-year follow-up period. Almost all people with HIV in this trial were receiving ART (61). Among household contacts of TB patients receiving TPT in the pre-HIV era, IPT had a long-lasting benefit, even in settings with very high rates of TB disease.
- The US Public Health Services sponsored several studies to assess the efficacy of IPT in the 1960s. A large group of individuals at risk for TB due to recent or remote contact with a pulmonary TB patient in Alaska were studied (114). In 1958, 2% of the population in this area was reported to have TB, and a tuberculin survey revealed an average annual rate of TB infection of 8%. These levels were among the highest ever reported, even higher than those in the highest-transmission settings, such as mines in South Africa, where an occurrence of 4.2% was estimated in 2005 (115). Participants received isoniazid at 300 mg daily or 5 mg/kg for children or a matching placebo for 1 year and were followed up actively for 2 years and passively for the next 10 years. Follow-up data from a study that started in 1958 of 28 villages and two boarding-schools in Alaska showed that the protective effect of isoniazid persisted for up to 19 years (116). The conclusion that 6–9 months of preventive therapy was optimal is based on follow-up data for this study and a study by the International Union Against Tuberculosis and Lung Disease that found that provision of isoniazid for more than 9 months does not increase its effectiveness (117,118).
- A systematic review published in 1999 (119) reaffirmed the effectiveness of isoniazid in preventing development of TB disease in approximately 60% of individuals in various at-risk groups, including family contacts. For every 35 recent household contacts with a positive TST who were prescribed isoniazid for 6 months, one case of TB disease was prevented during the next 5 years.
- A trial conducted in 2021 (120) clearly demonstrated that TPT was more likely to be completed if it included 3HP rather than 6H, and the protection provided by a single round of 3HP lasted as long as with isoniazid.
5.5 Repeating or re-starting TPT
No evidence is available on the utility of repeated courses of TPT, and WHO does not specifically recommend a repeated course of TPT. A randomized pragmatic trial (WHIP3TB) of people with HIV on ART in Ethiopia, Mozambique and South Africa, completed in late 2019, compared the effectiveness of 3HP given once (N=1802) or twice (N=1808) within 14 months versus one course of 6H (N=404) (120). Treatment completion was better with 3HP than 6H. Follow-up for 24 months after randomization showed similar rates of TB incidence, the incidence of rifampicin-resistant TB, and mortality in participants who received 3HP once or twice, suggesting that 3HP for people with HIV on ART in high TB transmission settings provides protection. An additional round of 3HP given approximately 1 year after the first did not provide additional benefit in preventing TB among people receiving ART, indicating that a single course of 3HP provides lasting protection. Longer follow-up of this trial will be important.
A repeated course of TPT should, however, be considered for people who previously completed a course of TPT but were subsequently in a household or in close contact with a TB patient. As the currently available tests (TST, TBST and IGRA) are not negative after a complete course of TPT, they cannot be used to determine eligibility for a repeated course in the case of a new exposure or reinfection. Careful assessment of the intensity of exposure and the balance between benefits and harm should guide a decision to administer a repeated course of TPT. TPT should also be considered for people with HIV who were previously treated for TB disease, especially infants and children. The risk of recurrence is likely to be determined by the extent of initial TB disease and the efficacy of previous TB treatment, which is substantially reduced by ART.
Re-starting TPT may be necessary if there has been a significant interruption. Section 7 proposes thresholds for determining whether to continue a regimen after interruption and how, largely based on criteria used in trials of different regimens. Reliable evidence on regimen interruption is lacking.
5.6 Does TPT cause drug resistance?
A common concern about wide-scale use of TPT is its potential to propagate drug resistance. While there is ample evidence that suboptimal treatment of TB disease favours the emergence of drugresistant TB strains, no convincing data are available of an association with TPT. A number of trials have failed to find evidence of a significant association between TB drug resistance and widescale use of isoniazid or rifamycin for TPT (121,122). Concerns such as these have deprived countless populations of the benefit of a potentially life-saving intervention.
An increase in drug resistance is unlikely if good TPT practices are observed by programmes, namely, that TPT is used for people without TB disease, the appropriate dosage is respected, and treatment is completed as prescribed. As individuals with TB infection have a small number of slowly replicating bacteria in their body, there is a low risk that TPT will select drug-resistant strains (121). It is in fact conceivable that TPT actually lowers the overall burden of TB disease and thus reduces the number of people among whom drug-resistant strains can emerge and spread.
TB disease should be excluded with all available tools before TPT is initiated, and regular follow-up done to ensure adherence to TPT and early identification of TB symptoms while on treatment. Drug susceptibility testing should be prioritized in individuals who develop microbiologically confirmed TB during or after TPT.
5.6.1 Isoniazid resistance after IPT
In a systematic review of 13 studies published between 1951 and 2006, which included 18 095 people on IPT and 17 985 controls, there was no suggestion of an increased risk of isoniazid-resistant TB after IPT (121). The results were similar when stratified for HIV. In addition, in the Thibela study cohort in South Africa, the proportions of TB episodes with drug resistance among patients who had received IPT did not significantly differ from those in comparison groups (122).
5.6.2 Rifamycin resistance after TPT
In an analysis of six RCTs of rifamycin-containing regimens for TPT versus active control or placebo, the occurrence of rifampicin resistance was 0.09% in 6808 individuals receiving rifamycin-based TPT vs 0.01% in 7415 individuals receiving alternative regimens (RR = 3.45, 95% CI 0.72 ; 16.56; P = 0.12) (121). In three of the studies in which intermittent rifamycin-based TPT was used, there were two cases of rifampicin resistance among 4673 individuals on an intermittent rifamycin-containing regimen and one case of rifampicin resistance among 4427 individuals on control regimens (RR = 3.89; 95% CI 0.44 ; 34.56; P = 0.22). In placebo-controlled trials, no cases of rifampicin resistance were found among participants receiving rifamycin-containing regimens, whereas several cases occurred in people on placebo (RR 0.20, 95% CI 0.02 ; 1.66) (122).
5.6.3 Resistance to levofloxacin after TPT
Microbiological sub-studies conducted within the V-QUIN and TB CHAMP trials provided no conclusive evidence of emergence of additional fluoroquinolone resistance in TB strains at the time of analysis in late 2023 (Annex 5 of (13)).
5.7 Introducing and scaling-up TPT
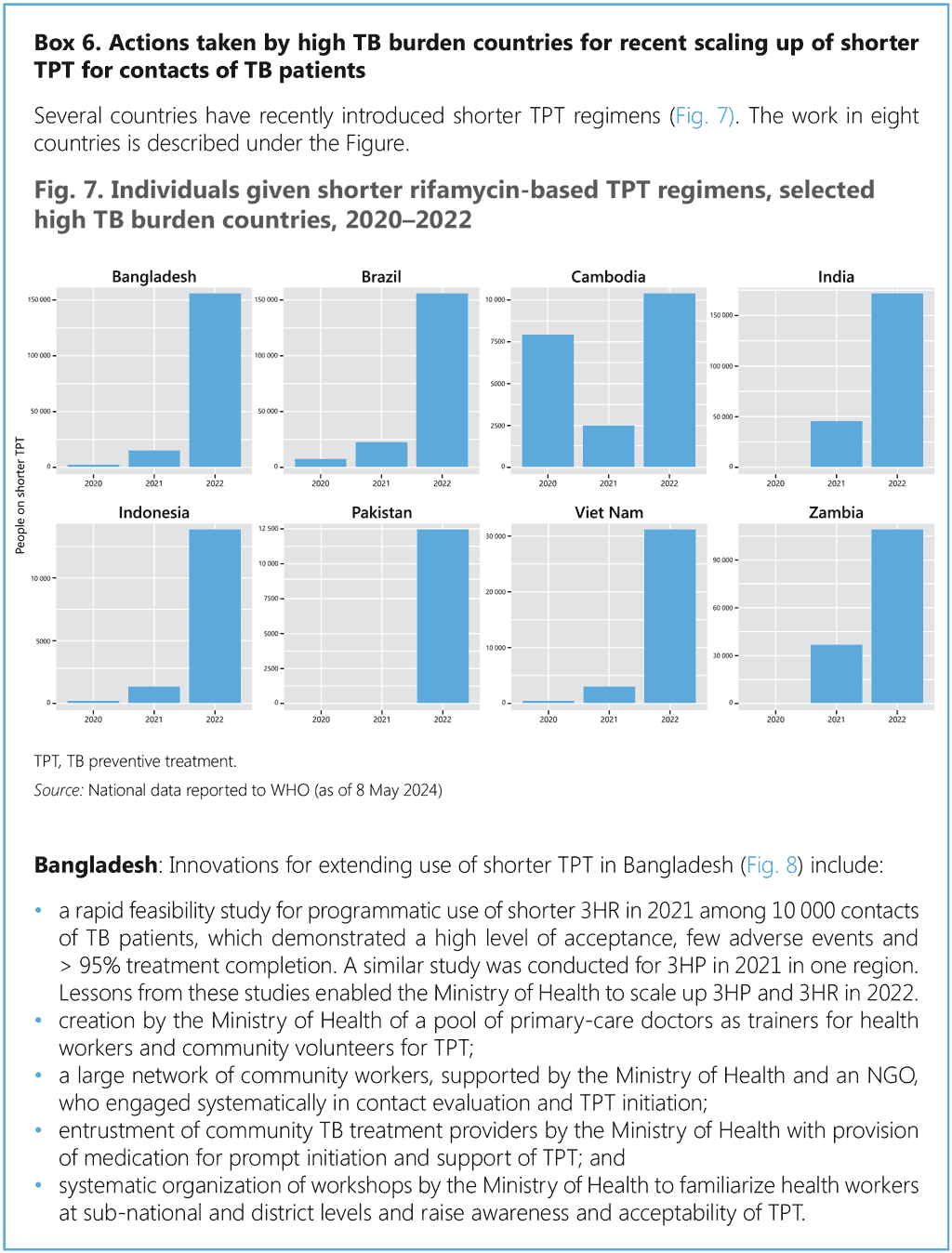
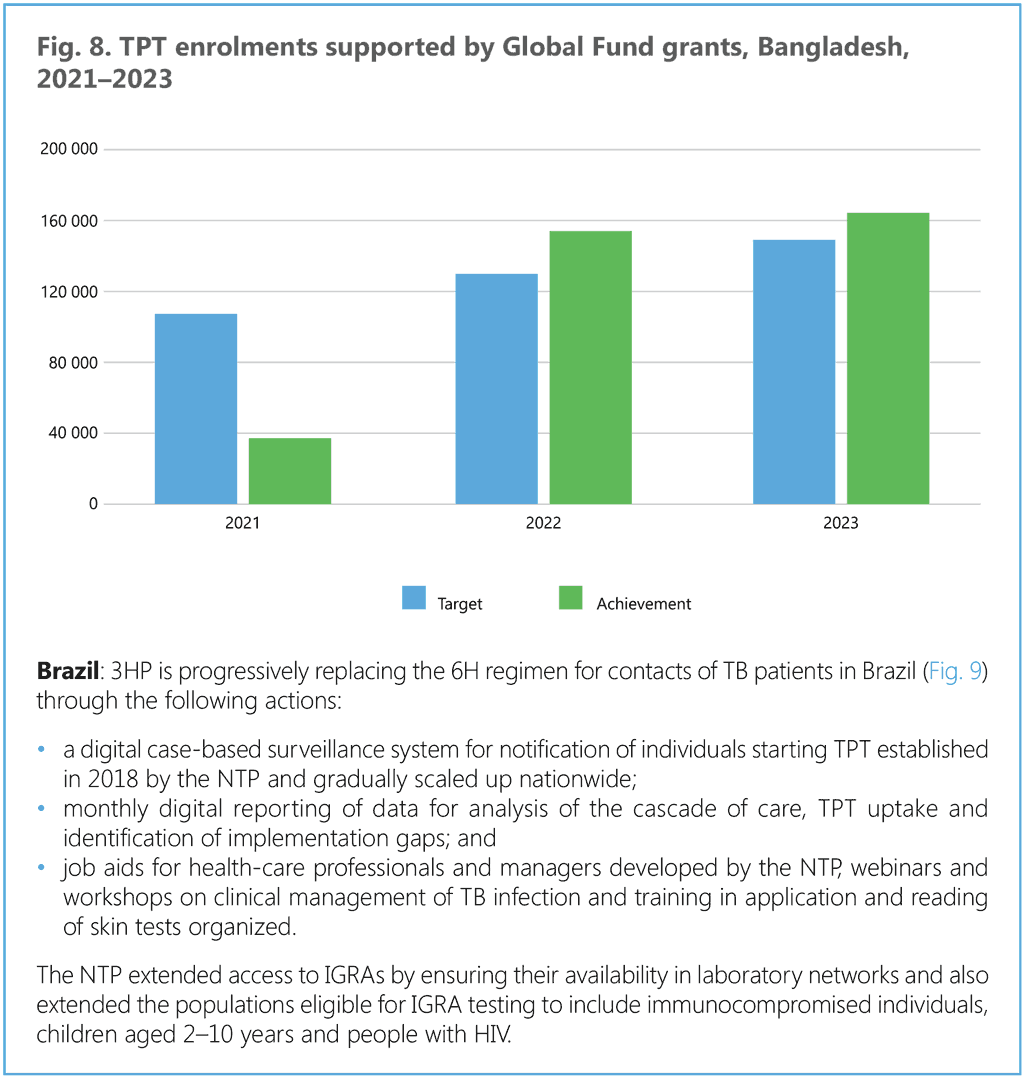
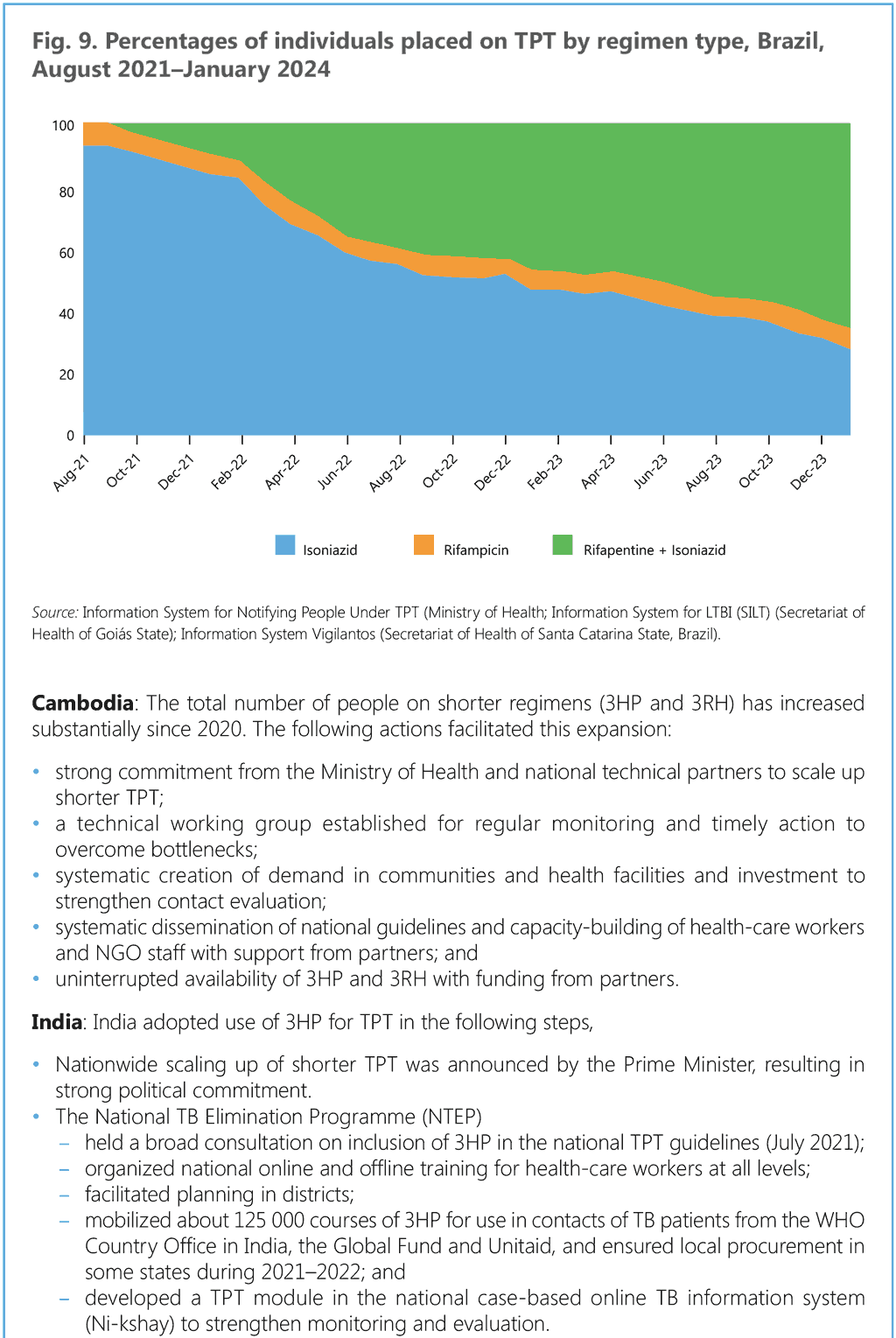
This section provides a stepwise approach to the introduction and extension of TPT in countries. Annex 3 provides additional details on coordination mechanisms for PMTPT. Box 5 provides an example from Brazil, where nurses have been entrusted with TPT administration through skill extension, and Box 6 illustrates actions taken by national programmes in high TB burden countries that have reported substantial increases in the use of shorter rifamycin-based TPT.
5.7.1 Considerations for programmatic implementation of TPT
- Define the roles, responsibilities and cadres of health-care workers in prescribing TPT. Trained doctors, nurses and peripheral health-care workers can evaluate and start TPT once TB disease has been reliably ruled out according to a national protocol. Nurses and front-line health-care workers can also be trained to monitor TPT and make decisions about whether TPT should be started, suspended, changed or re-started. This includes management of adverse events and treatment interruption. In most instances, it is unnecessary to seek the opinion of a medical doctor or a specialist for such decisions; however, provision for soliciting such support if it becomes necessary should be made.
- Define the levels of the health-care system at which TPT can be started and where refills of medicines can be accessed.
- Develop SOPs for TPT initiation and follow up to:
- maintain the flow of people identified for TPT among health facilities and service points in the facilities;
- ascertain the roles and responsibilities of health providers, community health workers and other stakeholders (such as nutrition care services, prisons and other correctional facilities, refugee camps, mining communities) in evaluation of eligibility and initiation of TPT;
- provide support for adherence to TPT;
- manage TPT interruptions; and
- identify, document and manage adverse drug events.
- Establish TPT services in all relevant delivery sites, such as TB treatment sites, ART centres, maternal and child health services and community health centres.
- Decentralize TPT to health facilities that initiate and continue TB treatment, to ART centres or to the facility closest to the person’s residence to minimize travel to receive TPT.
- Use existing TB, HIV and general health services to provide any specialized care required by people receiving TPT, such as management of severe or serious adverse events, drug–drug interactions, pregnancy and other special situations.
- Evaluate the capacity and availability of health-care workers, and assess additional requirements for nationwide scaling-up of TPT services.
- Evaluate the availability and capacity of community health workers and other networks. Former TB patients can contribute to TPT service delivery and support individuals and families in taking TPT.
- Build capacity through initial training, sensitization and mentoring of:
- primary care doctors, nurses and other health-care workers in taking a history, screening for symptoms, assessing eligibility for TPT, referral for investigations, conducting tests for TB infection and starting TPT; and
- community health workers in the provision of TPT and follow-up.
- Undertake phase-in/phase-out planning for TPT medications from a procurement perspective as the national programme changes to shorter TPT regimens. This is important during introduction of a new regimen.
- Review and strengthen the mechanism for quantification, ordering and an uninterrupted supply of TPT medications, pyridoxine and other commodities.
- Address specific issues regarding TPT for children, such as:
- coordinating TPT with several family members, including parents and grandparents, and target the many service delivery sites at which children receive care, such as maternal and child health services and TB and HIV centres;
- building capacity on managing vomiting of medication and indications for redosing; and
- providing information on foods that can mask the taste of medication.
- Strengthen systematic recording and reporting, including information from case forms, or capture data on electronic platforms. Data variables should be integrated into the HMIS for M&E of performance.

5.7.2 TPT initiation and pre-TPT baseline assessment
Once TB disease has been ruled out and a decision made to consider TPT, a baseline assessment should be made to determine the eligibility of an individual. In addition to testing for TB infection (as indicated), the baseline assessment includes a personal and medical history and investigations according to national guidelines.
- Personal history: elicit information relevant for TPT initiation and continuation, such as
- allergy or known hypersensitivity to TB drugs (isoniazid, rifampicin, rifabutin or rifapentine);
- HIV status and ART regimen;
- pregnancy status or birth control method used;
- comorbidity: presence of comorbidities (such as malnutrition, diabetes, viral hepatitis) and medications being taken;
- contacts of patients with drug-resistant TB (isoniazid, rifampicin only or MDR-TB); and
- potential contraindications to TPT: active hepatitis (acute or chronic) or known elevation in transaminases (more than three times the upper limit of normal), regular or heavy alcohol consumption and symptoms of peripheral neuropathy. These conditions should prompt detailed investigations and application of clinical judgement to weigh the harms against the benefits of TPT, and timing of the start of TPT if the benefits outweigh the harm. A history of TB treatment or current pregnancy should not be considered contraindications to starting TPT.
- History of medication: elicit a history of medication to guide the choice of TPT regimen or determine whether treatment of comorbid conditions should be modified. Certain drug classes – ARVs, opioids, antimalarials – often affect TPT
- Liver function test (LFT): There is insufficient evidence to support a mandatory or routine LFT at baseline (126) or whether the benefit of TPT without LFT would outweigh the harm, particularly with a less hepatotoxic regimen. When feasible, however, baseline testing is strongly encouraged for individuals with risk factors such as a history of liver disease, regular use of alcohol, chronic liver disease, HIV infection, age ≥ 35 years and pregnancy or immediately post partum (within 3 months of delivery). In individuals with abnormal baseline LFT results, sound clinical judgement is required to determine whether the benefit of TPT would outweigh the risk of adverse events. These individuals should be tested routinely at subsequent visits.
- The social and financial situation of the person and the family should be assessed and support provided to overcome barriers to TPT completion.
- Counselling: Explain to the individual that (s)he is eligible for TPT, and provide information to the individual, family and treatment supporter on the:
- rationale for TPT and the benefits to the individual, the household and the wider community;
- the availability of TPT free of charge through national programmes;
- the TPT regimen prescribed, including the duration, directions for intake of medicines and follow-up schedule;
- potential side-effects and adverse events and what to do if they occur;
- the importance of completing the full course of TPT;
- the reasons and schedule of regular clinical and laboratory follow-up for monitoring; and
- signs and symptoms of TB and advice on what to do if they occur.
Agree on the best approach to support treatment adherence, including the most suitable location for taking the drug and support according to each individual’s preference, such as:
- location: home, community or health facility (with counselling);
- treatment supporter: if required, could be an oriented family member, community volunteer, workplace colleague or health-care worker; in a weekly regimen, it is preferable that intake of each dose is directly observed by the supporter (either in person or with a digital tool); and
- digital tools: include video-supported treatment, electronic medication monitors and use of phone or text messaging to maintain contact with the individual or household.
Table A2.1 in Annex 2 summarizes the issues to be considered when deciding to start TPT.
5.7.3 Monitoring of adherence and treatment completion
Adherence to medication and treatment completion are important determinants of the clinical benefit of TPT. Individuals receiving TPT should therefore be well informed at every contact with healthcare providers. These elements are discussed further in section 7. Section 8 describes methods for monitoring and evaluating PMTPT in health services.
5.8 Differentiated HIV service delivery and implications for scaling up TPT
High HIV burden countries, particularly in sub-Saharan Africa, are increasingly scaling up differentiated HIV service delivery (DSD). DSD models for people with HIV who are stable on ART are personcentred, with the aim of changing treatment for people who are doing well to less intensive models, requiring fewer visits to health facilities. DSD is expected to reduce overcrowding at ART clinics, increase the quality of care, improve adherence and viral suppression rates and increase convenience. DSD includes appropriate support and education about potential adverse events, tolerability and the importance of treatment completion.
In principle, all TB services recommended for people with HIV, including regular TB screening, referral for diagnosis when TB symptoms are seen and TPT if TB disease is ruled out, should be included in DSD, and mechanisms for reviewing the quality of ART services should be used to monitor intensified TB case-finding and TPT services.
TPT may be started during an evaluation for ART or before starting spaced appointments under DSD, particularly for shorter regimens (1HP) or at the time of a follow-up visit to a health centre for longer regimens (6H, 6LFx, 4R, 3HR, 3HP) as per national guidelines. While TPT regimens of any length can be provided under DSD, it is critical to establish a mechanism for identifying and managing any adverse event, in view of the duration of TPT regimens, and to document TPT indicators systematically (see section 8). Fig. 10 illustrates the key elements of TPT to be integrated into DSD initiatives.
Fig. 10. Key elements of integration of TPT services into DSD models for ART
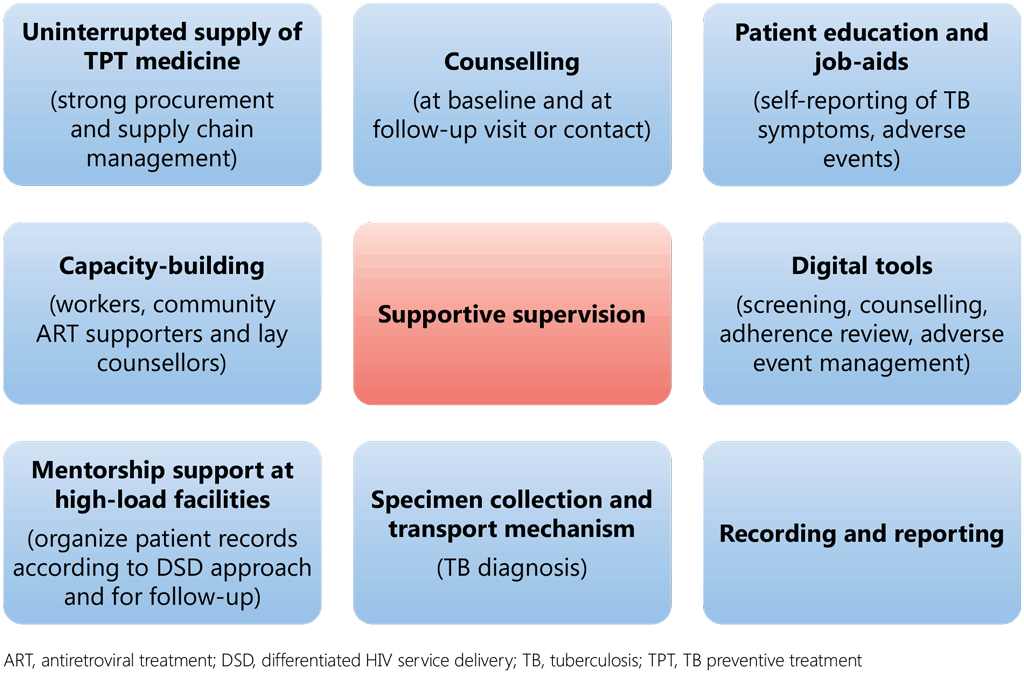
Various models of differentiated ART delivery have been tested, including health-care worker-managed group models; models used by people taking TPT; individual facility models; and community-based individual models. A few examples of implemented DSD models (127,128) are listed below.
- appointment spacing (facility and individual model): multi-month prescriptions and rapid circuit established at high-volume facilities to enable quick check-ups for stable patients and direct access to the pharmacy to collect medication;
- community points for ART distribution (community individual models): screening and drug distribution provided by lay health workers;
- adherence clubs (in facilities and communities): treatment distribution during support group meetings (every 3–6 months);
- specialized child-friendly clinics that provide 2–8 weeks of TPT and ART, depending on the travel schedule of each recipient; and
- remote access from commercial pharmacies or “pill boxes” (“medication ATMs”) without involvement of a community health-care worker
As more countries implement DSD, a key opportunity for scaling up TPT services is a country’s decision to change to new regimens, such as dolutegravir ART. Countries in eastern and southern Africa have decided to align their transition to a regimen comprising tenofovir, lamivudine and dolutegravir with scaling up of 3HP. As a transition to new ART requires more frequent clinical follow-up, national programmes are also using the opportunity to start TPT and to use the experience to guide national scaling up.
DSD models could also be used for household contacts of TB patients and other people without HIV who are nonetheless at increased risk of TB disease.
1 Isoniazid is metabolized by N-acetyltransferase 2 (NAT2), and a mutation in the NAT2 genotype leads to persistence of isoniazid in the body, predisposing to toxicity. The prevalence of NAT2 mutations differs geographically, with slow acetylators (known to be at risk of most drug-induced toxicity) very common in some settings (83% in Egypt and 67% in the USA) but rare elsewhere (12% in China).
 Feedback
Feedback