Book traversal links for 5.3.2.2. Shorter all-oral bedaquiline-containing regimen for multidrug-resistant and rifampicin-resistant TB in children
The standardized shorter all-oral bedaquiline-containing regimen may now be used in children of all ages under programmatic conditions.17 The eligibility criteria for this regimen for children with confirmed MDR/RR-TB are the same as for adolescents and adults:
- no resistance to fluoroquinolones;
- no previous exposure for more than 1 month to second-line medicines used in this regimen (unless susceptibility to these second-line medicines has been confirmed);
- no severe forms of EPTB (other than peripheral lymphadenopathy);
- no extensive TB disease (presence of cavities or bilateral disease on CXR);
- presence of mutations in both the inhA promoter region and the katG gene, as determined by firstline LPA (MTBDRplus) in the child or adolescent or their most likely source case, as this suggests that isoniazid at high dose and thioamides are not effective.
Children with a diagnosis of rifampicin resistance only, without further DST (such as a child diagnosed with Xpert MTB/RIF or Xpert Ultra testing on a stool sample but no further DST on respiratory samples), can be treated with available bedaquiline-containing regimens at the discretion of the treating clinician.
The standardized shorter all-oral bedaquiline-containing regimen18 is summarized as follows:
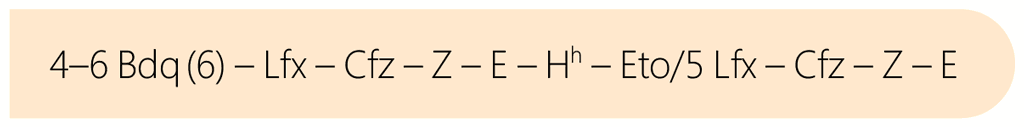
The regimen is shown in detail in Figure 5.2.
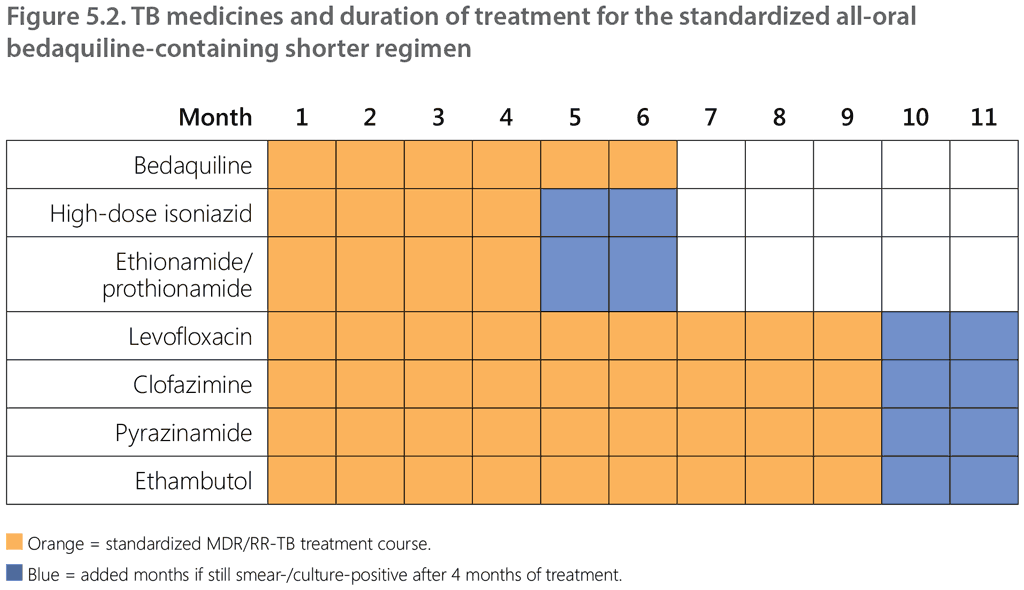
The medicines used in the standardized all-oral bedaquiline-containing regimen have been part of MDR/RR-TB regimens for many years, in similar combinations, for adults and children, except for bedaquiline, which was first recommended for use in adults in 2016 and in children aged over 6 years in 2019. Associated adverse reactions have been widely described (116), and the dosages established (see Annex 6). Dosages for bedaquiline in children and adolescents aged 0–17 years have recently been updated. For children aged under 6 years, these dosing recommendations are interim, as they were based on limited data and paediatric trials on bedaquiline are ongoing.
In children on the standardized all-oral bedaquiline-containing regimen who can produce a specimen, if smear or culture is negative at 4 months the treatment can be changed to the continuation phase. If smear or culture is positive at 4 months, the initial phase should be extended until smear or culture converts (maximum duration 6 months).
For children without bacteriological confirmation and those who cannot produce a specimen and who are clinically well, with improvement of clinical symptoms and weight gain, treatment can be changed to the continuation phase after 4 months (bedaquiline to be continued to 6 months).
Children who need an extension of the initial phase, who remain smear- or culture-positive, or who deteriorate further at 6 months should be switched to an individualized regimen. It is important to obtain follow-up specimens for culture and DST before changing treatment, but without waiting for these results. Results should be reviewed and treatment adjusted further as needed.
The pill burden of this regimen is relatively high, especially in the first 4–6 months, which may be challenging for young children, even if dispersible formulations are used. Treatment support is important to support administration.
17 New evidence on shorter all-oral bedaquiline-containing regimens will be reviewed by a WHO-convened guideline development group in 2022; therefore, this recommendation will soon be reviewed.
18 WHO published a rapid communication to announce forthcoming updates to treatment regimens for MDR/RR-TB in May 2022, including an alternative 9-month, all-oral, bedaquiline-containing regimen with linezolid instead of ethionamide. See: https://apps.who.int/iris/rest/bitstreams/1420701/retrieve
 Feedback
Feedback