Book traversal links for 1.1. Identifying populations for TB preventive treatment
Among individuals infected with M. tuberculosis, it is estimated that the average lifetime risk of progressing to TB disease is about 5–10% (4). The risk is particularly elevated among children under 5 years and among people with compromised immunity (1). As any treatment entails risk of harms and opportunity costs, TPT should be selectively targeted to population groups at highest risk of progression to TB disease, who would benefit most. When identifying populations at increased risk, consideration should be given to the epidemiology and pattern of TB transmission in the country, so that treatment is optimized to offer lasting protection. A comprehensive individual clinical assessment that considers the balance between the risks and benefits for the person receiving treatment is critical. This section describes recommendations for identifying population groups considered at highest risk of progression to disease and/or vulnerability to poor outcomes, namely people with HIV, contacts and other people at risk.
People with HIV
- Adults and adolescents living with HIV who are unlikely to have TB disease on an appropriate clinical evaluation or according to national guidelines should receive TB preventive treatment as part of a comprehensive package of HIV care. Treatment should also be given to those on antiretroviral treatment, to pregnant women and to those who have previously been treated for TB, irrespective of the degree of immunosuppression and even if testing for TB infection is unavailable. (Strong recommendation, high certainty of the estimates of effect)
- Infants aged < 12 months living with HIV who are in contact with a person with TB and who are unlikely to have TB disease on an appropriate clinical evaluation or according to national guidelines should receive TB preventive treatment. (Strong recommendation, moderate certainty of the estimates of effect)
- Children aged ≥ 12 months living with HIV who are considered unlikely to have TB disease on an appropriate clinical evaluation or according to national guidelines should be offered TB preventive treatment as part of a comprehensive package of HIV prevention and care if they live in a setting with high TB transmission, regardless of contact with TB. (Strong recommendation, low certainty of the estimates of effect)
- All children living with HIV who have successfully completed treatment for TB disease may receive TB preventive treatment. (Conditional recommendation, low certainty of the estimates of effect)
Justification and evidence
TB is the most frequent cause of AIDS-related deaths worldwide, despite progress in access to antiretroviral treatment (ART) (19). TB caused about 167 000 deaths among people with HIV in 2022, representing about one third of all HIV deaths (10). Globally, people with HIV are about 18 times more likely to develop TB disease than those without HIV infection.
Recommendation 1, to give TPT to all people with HIV, was first published by WHO in 2011 (20). A systematic review of 12 randomized controlled trials (RCTs) found that preventive treatment reduced the overall risk for TB by 33% (relative risk [RR] 0.67, 95% confidence interval [CI] 0.51; 0.87) among the 8578 people with HIV included in the trial (21). For those who were tuberculin skin test (TST) positive, the reduction increased to 64% (RR 0.36, 95% CI 0.22; 0.61). Although not statistically significant, the reduction was 14% among TST-negative people (RR 0.86, 95% CI 0.59 ; 1.26) and those of unknown TST status (RR 0.86, 95% CI 0.48 ; 1.52). Most of the studies in the review were, however, conducted before ART became available, and there is now increasing evidence from observational studies and RCTs of the efficacy of TPT in people receiving ART. TB incidence has been reported to be high among all people with HIV who did not receive isoniazid preventive treatment (IPT), including those with a CD4 cell count > 350/mm3 and who were TST negative (22). A double-blinded RCT of 1329 people with HIV receiving ART found that the effect of IPT was not statistically significantly different between those who were positive or negative on TST or IGRA (23). An RCT of 2056 people with HIV showed additive benefits of TPT plus ART in reducing both TB incidence and overall mortality (24,25). Early initiation of ART and 6 months of IPT independently resulted in a risk of severe HIV-related illness that was 44% lower and a risk of death from any cause that was 35% lower than the risks with deferred initiation of ART and no IPT. The protective effect lasted for > 5 years.
The GDG at that time reviewed the evidence from the systematic reviews and discussed each population risk group identified for the prevalence of TBI, risk of progression to TB disease and the incidence of TB disease as compared with that in the general population. They concluded that the evidence shows a clear benefit of systematic testing and treatment of TBI for people with HIV. The wording of the current recommendation refers to TBI testing rather than TST as IGRA, and the new antigen-based skin tests (TBST) are alternative options (see recommendations 17 and 18). TPT should be given to adults and adolescents with HIV, regardless of their immune status and whether they are on ART, given the evidence of a protective effect additional to that of ART. A systematic review of studies conducted before ART became available showed the value of providing TPT immediately after successful completion of TB treatment among people with HIV in countries with a TB incidence > 100/100 000 population (26,27). Since 2011, TPT has been recommended for children with HIV who were previously treated for TB (see next section). No evidence was found, however, for preventive treatment of people who had successfully completed treatment for MDR- or extensively drug-resistant TB. The effect of repeated courses of TPT is also unclear due to lack of evidence, and hence no recommendation was made (28). The relative risk of TB transmission is determined by local authorities on the basis of risk of exposure (e.g. TB incidence, occurrence of undiagnosed or inadequately treated disease, population density, environmental factors) and host immune response (29).
Pregnant women with HIV are at risk for TB, which can have severe consequences for both the mother and the fetus, with increased risks of maternal and infant death (30). Pregnancy should not disqualify women with HIV from receiving TPT with medicines commonly used to treat TB disease that are generally considered safe for use in pregnancy, such as isoniazid and rifampicin (classified as pregnancy category C by the US Food and Drug Administration (31,32)). Section 1.4 presents the position on use of TPT in pregnancy.
Recommendations 2–4 were first published by WHO in 2011 (20). A systematic review conducted for establishing the original guidelines included two studies, both conducted in South Africa. One suggested a considerable reduction in mortality and protection against TB among HIV-infected children who received isoniazid for 6 months (33). The other, however, showed no benefit of preventive treatment in infants in whom HIV infection was identified in the first 3–4 months of life, who had no known exposure to TB disease and who were rapidly placed on ART and monitored carefully every month for new exposure to TB or emergence of TB disease (34). Few RCTs included children on ART. In one trial of 167 children on ART, the incidence of TB was lower in those given TPT than in those who were not, but the difference was not statistically significant (incidence rate ratio 0.51, 95% CI 0.15; 1.75) (35). A cohort study suggested an additive protective effect of TPT in children receiving ART (36).
For infants with HIV aged < 12 months, the GDG recommended that TPT be given only to those who have a history of household contact with a person with TB and are considered not to have TB disease according to investigations conducted in line with national guidelines, because of limited data on the benefits. The GDG strongly recommended TPT for children aged ≥ 12 months with HIV but without clinical manifestations suggestive of TB disease, despite the low certainty of the evidence, because of the clear benefits seen in adults with HIV and the high risk for TB disease among people with HIV. Children ≥ 12 months with HIV who have clinical manifestations or who are contacts should be evaluated further and treated for TB disease or TBI as indicated (see also Fig. 1).
The GDG noted that, although the evidence for the efficacy of TPT in children on ART is limited, it is biologically plausible, given the evidence of additive effects in adults with HIV receiving ART. Thus, TPT is recommended for children, regardless of whether they are on ART or not.
Despite limited evidence on the value of TPT in children with HIV after successful completion of TB treatment (20), the GDG considered that children with HIV who are at risk of reinfection could benefit from TPT. Therefore, the GDG conditionally recommended that all children with HIV who have been successfully treated for TB and are living in settings with high TB transmission as defined by national authorities (see also Definitions) may receive a course of TPT. This can be started immediately after the last dose of TB curative treatment or later, according to clinical judgement.
Household contacts of people with TB, regardless of HIV status
- Children aged < 5 years who are household contacts of people with bacteriologically confirmed pulmonary TB and who are found not to have TB disease on an appropriate clinical evaluation or according to national guidelines should be given TB preventive treatment even if testing for TB infection is unavailable. (Strong recommendation, high certainty of the estimates of effect)
- Children aged ≥ 5 years, adolescents and adults who are household contacts of people with bacteriologically confirmed pulmonary TB who are found not to have TB disease on an appropriate clinical evaluation or according to national guidelines may be given TB preventive treatment. (Conditional recommendation, low certainty of the estimates of effect)
Justification and evidence
Recommendation 5 was initially published by WHO in 2015 and recommendation 6 in 2018 (17,37). A systematic review conducted for the 2015 guidelines on household contacts in countries with a TB incidence > 100/100 000 population was updated in 2018 (37–39) (see PICO 1 in Annex 3). The aim of the review was to determine the prevalence of TBI, progression to TB disease and the cumulative prevalence of TB among household contacts, stratified by age. Another 19 studies published between 2014 and 2016 were added. While the evidence reviewed related to HIV-negative child contacts, children with HIV who are household contacts of a person with bacteriologically confirmed pulmonary TB should also undergo investigation and treatment as necessary.
The prevalence of TBI was higher among adolescents aged > 15 years and adults than in children < 5 years, who were at greatest risk for progression to TB disease. In comparison with child household contacts < 5 years, the pooled risk ratios for progression to TB disease were lower in children aged 5–15 years (0.28, 95% CI 0.12 ; 0.65, four studies) and for those aged > 15 years (0.22, 95% CI 0.08 ; 0.60, three studies). All household contacts, regardless of their age or TBI status, were at substantially higher risk for progression to TB disease than the general population (Table 2).
Table 2. Pooled estimates of risk for TB disease among household contacts stratified by age and baseline TBI status as compared with the general population
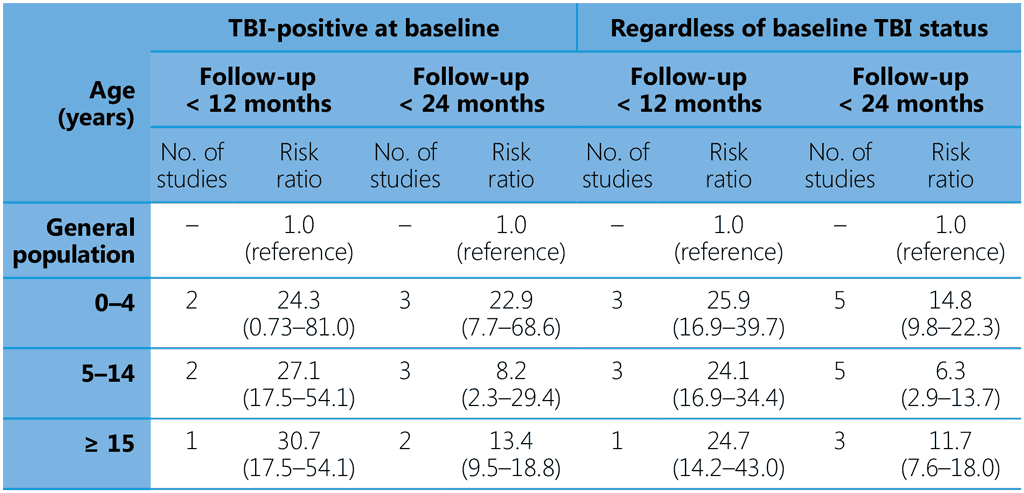
Both recommendations may apply to people with or without HIV. The GDG noted the significantly higher risk of infants and young children < 5 years for developing TB. Furthermore, the disease can develop rapidly in young children, and they are at greatest risk of severe and disseminated disease, which are associated with high morbidity and mortality. Therefore, the GDG strongly recommended TPT for child household contacts aged < 5 years, regardless of HIV status and background epidemiology of TB, but only after TB disease has been ruled out.
TPT is also conditionally recommended for household contacts in other age groups, according to clinical judgement on the balance between harm and benefit for individuals and the national and local epidemiology of TB, with special consideration of ongoing transmission of TB. In this group, confirmation of TBI with either IGRA or a skin test would be desirable (see section 1.3). With evidence of moderate to high certainty, the 2015 guidelines strongly recommended systematic testing and treatment of TBI in contacts, regardless of age, in countries with a TB incidence < 100/100 000 population (37). In the 2020 update, the GDG considered that this recommendation could be applied in any country regardless of TB burden if tests for TBI and tests to rule out TB are available and reliable. Treatment may be justifiable without a TBI test after an assessment of the individual’s risk of exposure and for development of TB disease in a given setting. The GDG noted that important considerations in implementation of these recommendations are the capacity of a caregiver to assess the intensity of exposure, the risks of infection and reinfection, the risk for developing TB disease and ascertainment of TBI by testing, as well as capacity to weigh the harm versus the benefit of treatment and the ability to exclude TB disease before initiation of treatment.
Other people at risk
- People who are initiating anti-tumour-necrosis factor treatment, receiving dialysis, preparing for an organ or haematological transplant or who have silicosis should be systematically tested and treated for TB infection. (Strong recommendation, low to very low certainty of the estimates of effect)
- Systematic testing and treatment for TB infection may be considered for prisoners, health workers, immigrants from countries with a high TB burden, homeless people and people who use drugs. (Conditional recommendation, low to very low certainty of the estimates of effect)
Justification and evidence
Recommendations 7 and 8 were first published by WHO in 2015 (37). The GDG considered evidence from three systematic reviews that were conducted for the previous guidelines on TBI to determine which of the 24 defined at-risk population groups should be prioritized for TBI testing and treatment (37–39). Evidence of an increased prevalence of TBI, an increased risk of progression from TBI to TB disease and an increased incidence of TB disease was available for the following 15 risk groups: adult and child TB contacts, health-care workers and students, people with HIV, patients on dialysis, immigrants from countries with a high TB burden (incidence > 100 TB cases per 100 000 population), patients initiating anti-TNF therapy, people who use drugs, prisoners, homeless people, patients preparing for an organ or haematological transplant, patients with silicosis, patients with diabetes, people who engage in harmful use of alcohol, tobacco smokers and underweight people (38). An increased risk for progression to TB was reported for 4 of the 15 groups: people with HIV, adult and child TB contacts, patients on dialysis and underweight people.
The GDG judged that people in clinical risk groups, such as patients initiating anti-TNF treatment, patients on dialysis, patients preparing for organ or haematological transplant and patients with silicosis (40), would benefit most from testing for and treatment of TBI, regardless of the background TB epidemiology. The GDG considered that the benefit of TPT in reducing the risk of progression to disease would usually outweigh potential harm in these groups and made a strong recommendation despite low to very low certainty of the evidence.
The GDG concluded from the evidence that the benefits of systematic testing for TBI and TPT may not always outweigh the harm in health-care workers and students, immigrants from countries with a high TB burden, prisoners, homeless people and people who use drugs. The GDG judged, however, that the benefits are more likely to outweigh potential harm when the risks for reinfection are lower. In 2020, the GDG updated this recommendation to make it applicable to countries with both high and low TB prevalence on condition that a decision for systematic testing for TBI and offering TPT in these population groups be based on the local TB epidemiology and context, health infrastructure, capacity to exclude TB disease reliably, any adverse impact on health equity and overall health priorities. Greater benefit is expected for individuals who were recently infected with TB, as documented by conversion from a negative to a positive test of TBI (see section 1.3). The GDG also concluded that recent immigrants, particularly those from countries with a higher TB burden than that in the host country,3 may be prioritized, especially within the first few years after entry.
Despite evidence for increased prevalence of TBI and disease in patients with diabetes, people who engage in harmful use of alcohol, tobacco smokers and underweight people, the GDG in 2014 noted the paucity of data from clinical trials on the benefits and harm of systematic testing and treatment of TBI. They concluded that systematic, routine testing and treatment of people with these risks alone might not always outweigh the potential harm, regardless of background TB epidemiology. In 2014, a recommendation against systematic testing and treatment of TBI in these four populations was issued due to the lower risk of progression from infection to disease than in the other at-risk populations listed above, in whom TPT was recommended. This was not based on direct evidence that TPT is harmful but was rather an attempt to prioritize TPT for populations at the highest risk of progression to disease. The recommendation was not intended to be construed as a blanket recommendation against any testing or treatment in these populations but rather for a case-by-case assessment of risk. Regrettably, the recommendation was often misinterpreted as meaning that diabetes, use of alcohol, tobacco smoking and underweight were contraindications for TPT in individuals who were otherwise eligible. Thus, in 2024, the GDG reconsidered its position and replaced the recommendation with a statement that no recommendation is possible for these subgroups, given the evidence. Trial evidence on TPT in people with diabetes is expected to become available for review in a few years’ time (42).
The GDG agreed that prioritization of groups according to their risk and the local and national context would be acceptable to people with TBI and to stakeholders such as clinicians and programme managers. It noted that the high risk for ongoing TB transmission in certain groups, such as front-line health-care workers (including students), prisoners (and prison staff), immigrants from areas with a higher TB burden than that in the host country, homeless people and people who use drugs, requires attention, so that the benefit of treatment is not compromised by subsequent reinfection. TPT complements other preventive components of the programme for active TB case-finding, infection control and early treatment of TB disease (29).
Implementation considerations
In their normative and planning documents, national TB and HIV authorities and other stakeholders should clearly define priority populations for PMTPT. The aim should be to provide lasting protection from progression to TB disease to a maximum number of individuals at risk, thus limiting continued transmission and reinfection and reducing TB incidence over time. People with HIV and household contacts were the primary targets for global action by Member States at the United Nations high-level meetings in 2018 and 2023 (6,43). The GDG stressed that the best available evidence should be used to ensure that the benefits outweigh the risks to individuals in these groups and to make the best possible use of resources, which could yield savings for the entire health-care system. Any additional resources necessary to implement the guidance should not be viewed as a barrier but should stimulate programmatic mobilization of more funding. The GDG noted the value of ART in preventing TB in people with HIV and urged countries to ensure universal access to ART, as per WHO policy (44).
Provision of TPT for people with HIV should be a core component of the HIV package of care and should be the responsibility primarily of national HIV/AIDS programmes and HIV service providers (44,45). Some household contacts and other people eligible for TPT (e.g. people receiving dialysis, prisoners) will also be HIV positive and would therefore require individual attention to minimize the likelihood of developing TB disease. Care should be coordinated with the health services responsible for TB. TPT should be viewed as one of a comprehensive set of interventions. Among people with HIV who were treated for TB in the past, TPT should be prioritized for adults and adolescents who have been re-exposed to TB.
In addition to HIV care, nutrition supplementation has been shown to reduce the risk of TB disease by 39–48% in household contacts who are undernourished (46).
Confirmation of TBI with either IGRA or skin testing and reliable exclusion of TB disease with sensitive tests such as CXR are desirable before starting TPT. If these tests are not available, TPT should not be withheld from eligible people if TB disease has been excluded on clinical grounds alone (see section 1.2).
Identification of populations for TBI testing and TPT raises various ethical issues (47,48). First, as TBI is an asymptomatic, non-contagious state, the ethical obligations are different from those for TB disease. For example, in the absence of an immediate risk of transmission, it would be unethical to restrict the movement of a person with TBI who refuses treatment. Lack of evidence of the benefit of systematic testing and treatment in certain populations (e.g. people with diabetes or who are underweight) should not preclude offering preventive treatment to individuals with these conditions who are judged to be at increased risk of progression. Secondly, lack of tests for measuring individual risk for development of TB disease may complicate communication. Informed consent requires effective, adequate communication to each person about the uncertainty of current TBI tests to predict progression to TB disease, individual host variation and the protective benefit expected from treatment versus adverse reactions. Appropriate means to obtain informed consent should comply with international human rights standards and account for differences in language, literacy and legal status. Risk and uncertainty must be communicated in a way that is culturally and linguistically appropriate, including to people whose first language is not that of the local setting, to children and to people in prison. User feedback collected during screening programmes is useful for communication. Thirdly, TBI disproportionately affects individuals and groups that are already disadvantaged due to factors such as disease, socio-economic situation or legal status. Efforts must be made to address any inequity in access to services and to uphold human rights, so that the vulnerability of target groups does not impede their access to screening and treatment or violate their rights. Any intervention for vulnerable groups, including people in prisons and children, should include measures to minimize the risk of stigmatization, such as protecting confidentiality of personal data and informed consent. The GDG emphasized that a person’s status – positive for TBI or receiving TPT – should not affect any immigration procedure or entry to the host country, and this should be reflected in laws or other regulations. People should be tested for TBI and receive TPT in strict adherence to human rights and ethical considerations (49). Policies should be evaluated by users from an ethical perspective and the views and experiences of affected populations collected after implementation, both to consider possible unexpected effects and to ensure that the evidence on which they are based remains current and relevant (50). Person-centred TBI care includes equitable provision, with no added disadvantage for marginalized and vulnerable populations, and emphasizes the human rights aspects of TPT so that appropriate safeguards are included in law, policy and practice to minimize any additional stigmatization, discrimination, violation of bodily integrity or restrictions on freedom of movement. In person-centred TBI care, people who are offered testing and treatment must understand the uncertainties, so that they can participate in care options. These guiding principles are based on established principles of human rights such as consent, non-coercion and confidentiality (48).
3 Estimated rates of TB incidence in all countries are updated annually by WHO (41)
 Feedback
Feedback