Перекрёстные ссылки книги для A2.5 Information sheet: Practical considerations for implementation of the Cepheid Xpert MTB/XDR test
The Xpert MTB/XDR detects Mycobacterium tuberculosis complex (MTBC) DNA and genomic mutations associated with resistance to isoniazid (INH), fluoroquinolones (FQs), ethionamide (ETH) and second-line injectable drugs (amikacin [AMK], kanamycin and capreomycin) in a single cartridge. Xpert MTB/XDR tests are run on Cepheid's GeneXpert instruments, using 10-colour modules that differ from the 6-colour modules traditionally used for Xpert MTB/RIF and Xpert MTB/RIF Ultra (Xpert Ultra) testing. Xpert MTB/XDR is intended for use as a reflex test in tuberculosis (TB) specimens (unprocessed sputum or concentrated sputum sediments) determined to be MTBC-positive (1). These tests have been assessed in various studies (2-4). The World Health Organization (WHO) includes this test within the class of low complexity automated nucleic acid amplification tests (NAATs), and the recommendations below apply to this test (6, 7).
WHO recommendations for use
WHO recommends the use of low complexity automated NAATs in the following situations (8):
- In people with bacteriologically confirmed pulmonary TB, low complexity automated NAATs may be used on sputum for initial detection of resistance to INH and FQs, rather than culture-based phenotypic drug-susceptibility testing (DST).(Conditional recommendation; moderate certainty of evidence for diagnostic accuracy)
- In people with bacteriologically confirmed pulmonary TB and resistance to RIF, low complexity automated NAATs may be used on sputum for initial detection of resistance to ETH, rather than DNA sequencing of the inhA promoter(Conditional recommendation; very low certainty of evidence for diagnostic accuracy)
- In people with bacteriologically confirmed pulmonary TB and resistance to RIF, low complexity automated NAATs may be used on sputum for initial detection of resistance to amikacin, rather than culture-based phenotypic DST.(Conditional recommendation; low certainty of evidence for diagnostic accuracy)
There are several subgroups to be considered for these recommendations:
- The recommendations are based on the evidence of diagnostic accuracy in sputum of adults with bacteriologically confirmed pulmonary TB, with or without RIF resistance.
- The recommendations are extrapolated to adolescents and children, based on the generalization of data from adults.
- The recommendations apply to people living with HIV (People with HIV) (studies included a varying proportion of such people); data stratified by HIV status were not available.
- The recommendations are extrapolated to people with extrapulmonary TB, and testing of non-sputum samples was considered appropriate, which affects the certainty. The panel did not evaluate test accuracy in non-sputum samples directly, including in children; however, extrapolation was considered appropriate given that WHO has recommendations for similar technologies for use on non-sputum samples (e.g. Xpert MTB/RIF and Xpert Ultra).
- Recommendations for detection of resistance to AMK and ETH are only relevant for people who have bacteriologically confirmed pulmonary TB and resistance to RIF.
Key performance conclusions
- Xpert MTB/XDR assay performs well as a follow-on test for patients with bacteriologically confirmed TB for the detection of resistance to INH, FQ, ETH and AMK.
- Limit of detection as reported by the manufacturer for TB detection is 136 colony forming units (CFU)/mL for unprocessed sputum and 86 CFU/mL for sputum sediment.
- The pooled sensitivity and specificity data for the class are presented in the Web Appendix of the WHO consolidated guidelines (8).
Test procedure at-a-glance
The sample processing procedure and cartridge handling are the same as for Xpert MTB/RIF and Ultra tests. However, the Xpert MTB/XDR test runs on a GeneXpert platform that supports multiplexing via 10-colour technology, instead of the 6-colour technology used with Xpert MTB/RIF and Ultra tests.
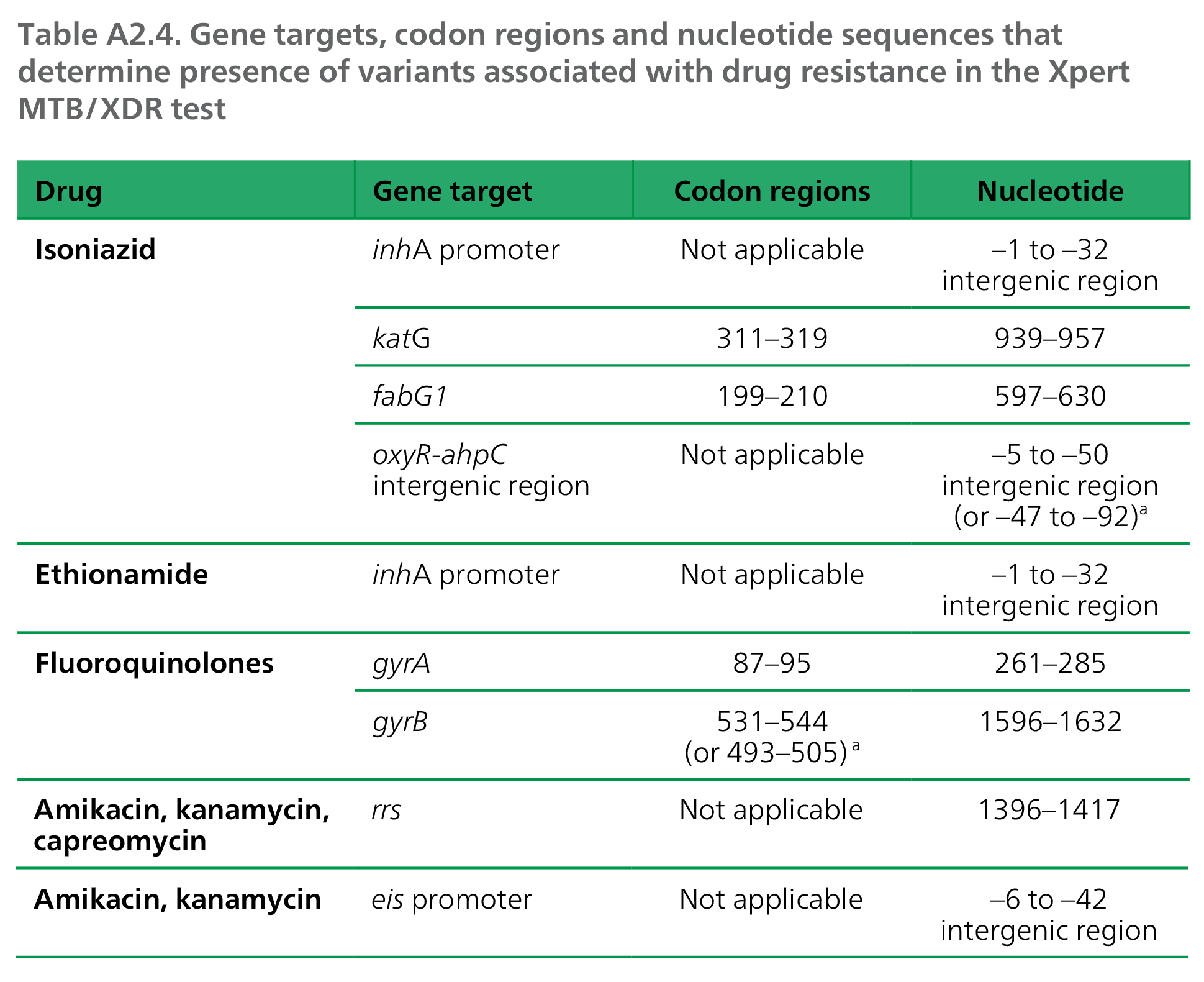
The test targets the genes, codon regions and nucleotide sequences given in Table A2.4.
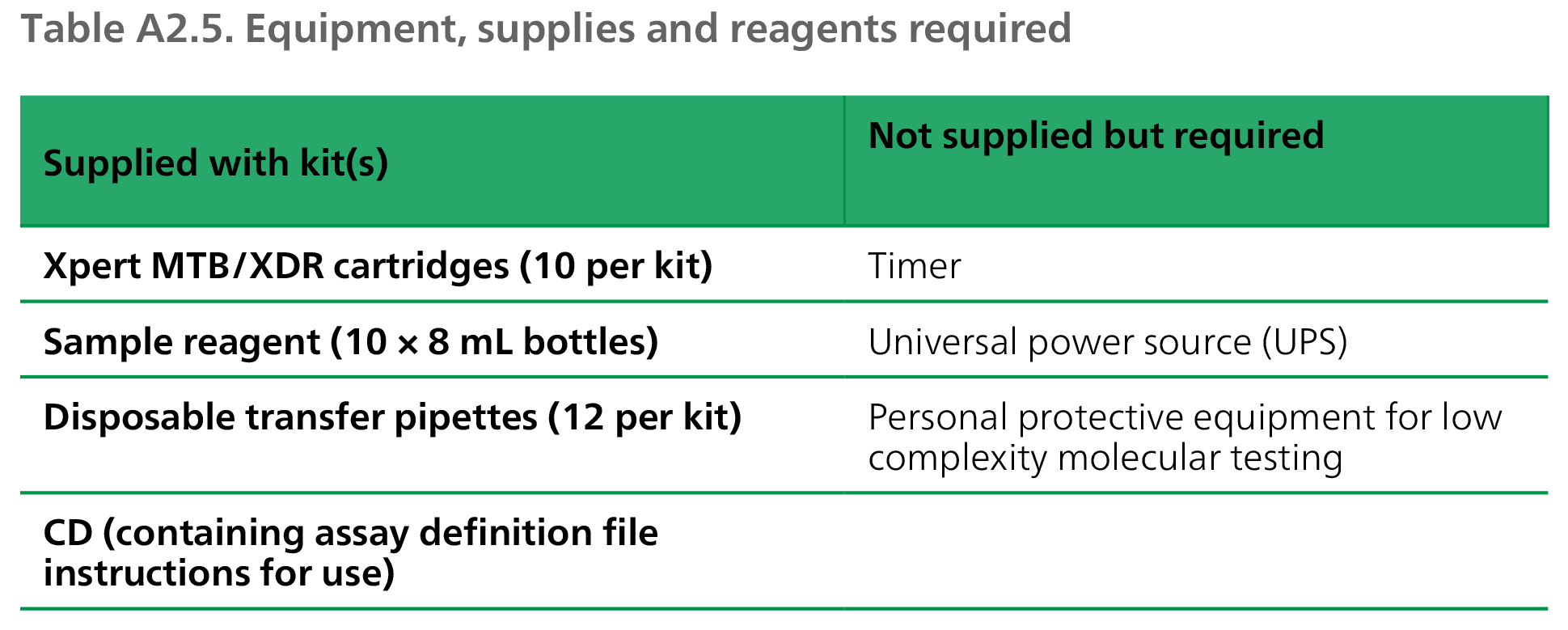
Equipment, supplies and reagents

10-colour GeneXpert module(s): Available for GeneXpert instrument models GX I to GX XVI (Fig. A2.2) may be procured as new modules, new systems (i.e. instrument, computer or laptop, or barcode scanner) or as satelites (instrument only) to connect to existing GeneXpert systems. There is also the possibility to convert an existing GeneXpert system with 6 colours modules to 10 colour modules, but hybrid 6 and 10-colours instruments are not supported by Cepheid for now.
Operational considerations
- Testing capacity: Maximum of five 90-minute tests per module in an 8-hour workday (10).
- GeneXpert instrument service and maintenance: Various options are offered by the manufacturer.
- Sputum storage and testing conditions: Testing may be conducted on raw sputum stored at 2-35 °C for no more than 7 days or sputum sediment stored at 2-8 °C for no more than 7 days. After addition of sample reagent, test within 2.5 hours if sample is stored at up to 35 °C or test within 4 hours if it is kept at 2-8 °C (10).
- Storage temperature: Xpert MTB/XDR cartridges and reagents (2-28 °C) and 10-colour GeneXpert instruments (≤30 °C).
- Unit prices for low- and middle-income countries: Xpert MTB/XDR cartridges cost US$ 19.80 per test or US$ 198.00 per kit (10 tests) (8). 10-colour GeneXpert modules range from US$ 3860 (single module kit) to US$ 72 350 (new GX XVI system) (11).
Implementation considerations
In addition to general guidance provided in Section 3.5, consider the following test-specific implementation considerations:
- Area 1 - Policies and planning: Xpert MTB/XDR integration into national algorithms and network placement should consider that the test may be placed as low as near-point-of-care settings, and that patient specimens may need to undergo further testing such as culture or DST (e.g. to detect resistance to bedaquiline, delamanid or other medicines (8)). Because this test may primarily be used as a follow-on test for laboratory-confirmed TB, budget planning for test implementation should consider test volumes as well as strategic procurement of 10-colour modules, and instruments or systems that leverage or complement existing GeneXpert networks.
- Area 3 - Equipment: Xpert MTB/XDR cartridges are only compatible with 10-colour GeneXpert modules and will not function on the 6-colour modules traditionally used for Xpert MTB/RIF and Xpert Ultra testing.
- Area 6 - Digital data: GeneXpert instruments with 10-colour modules have the same diagnostic connectivity opportunities as instruments with 6-colour modules, allowing for SMS-based and e-based transmission of results and other connectivity application features, such as commodity and quality monitoring. The assay results could be transferred to laboratory information systems (LIS), as with previous TB Xpert tests. The addition of the Xpert MTB/XDR test to your LIS will probably require a collaboration with the LIS provider to implement the new test settings because the result reporting will be different from that of currently used TB Xpert assays.
- Area 7 - Quality assurance: Quality assurance systems and activities for Xpert MTB/ XDR mimic those for Xpert MTB/RIF (12) or Xpert Ultra, with a few differences based on differences in test design. Given inclusion of an expanded set of probes for detection to a wider range of TB medicines, new method verifications should be conducted using a panel of well-characterized M. tuberculosis strains, such that sensitivity and resistance for each TB medicine included in the assay (RIF, INH, FQ, ETH and AMK) is represented, to demonstrate that the laboratory can achieve expected accuracy and precision of resistance detection. Once completed, decentralized testing sites do not need to repeat a new method verification with a comprehensive resistance panel unless required by national policy or one or more accrediting body, but should conduct new method verifications, as was done for the introduction of Xpert MTB/RIF or Xpert Ultra tests. Post-market validation and new-lot testing should be conducted according to established TB Xpert protocols.
- Area 8 - Recording and reporting: Given the potential for decentralized testing sites to report an expanded set of drug-susceptibility results for the first time, countries should consider whether any additional communication routes or reporting procedures may be required. In addition, diagnostic connectivity solutions may be used to automate reporting. Revisions to laboratory registers and reporting forms may be needed.
- Area 9 - Training and competency assessment: Training on new cartridges should be incorporated into current refresher training programmes as well as pre-implementation sensitization on the technology updates. As with other molecular DST, laboratory staff and clinicians should be trained on appropriate review and interpretation of resistance results for all included medicines.
References for A2.5
- Cepheid. Package insert portal [website]. 2020 (https://www.cepheid.com/en_US/package-inserts/1615).
- Cao Y, Parmar H, Gaur RL, Lieu D, Raghunath S, Via N et al. Xpert MTB/XDR: a 10-color reflex assay suitable for point-of-care settings to detect isoniazid, fluoroquinolone, and second-line-injectable-drug resistance directly from Mycobacterium tuberculosis-positive sputum. Journal of clinical microbiology. 2021;59(3):e02314-20.
- Penn-Nicholson A, Georghiou SB, Ciobanu N, Kazi M, Bhalla M, David A et al. Clinical evaluation of the Xpert MTB/XDR assay for rapid detection of isoniazid, fluoroquinolone, ethionamide and second-line drug resistance: A cross-sectional multicentre diagnostic accuracy study. medRxiv. 2021;(https://www.medrxiv.org/content/10.1101/2021.05.06.21256505v1).
- Bainomugisa A, Gilpin C, Coulter C, Marais BJ. New Xpert MTB/XDR: added value and future in the field. Eur Respiratory Soc. 2020.
- Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio. 2017;8(4):e00812-17 (https://pubmed.ncbi.nlm.nih.gov/28851844).
- WHO consolidated guidelines on tuberculosis Module 4: treatment - drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2020 (https://www.who.int/publications/i/item/9789240007048).
- Update on the use of nucleic acid amplification tests to detect TB and drug-resistant TB: rapid communication. Geneva: World Health Organization; 2021 (https://www.who.int/publications/i/item/update-on-the-use-of-nucleic-acid-amplification-tests-to-detect-tb-and-drug-resistant-tb-rapid-communication).
- WHO consolidated guidelines on tuberculosis Module 3: diagnosis - rapid diagnostics for tuberculosis detection. Geneva: World Health Organization; 2021 update (https://apps.who.int/iris/bitstream/handle/10665/342331/9789240029415-eng.pdf).
- Camus J-C, Pryor MJ, Medigue C, Cole ST. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiol. 2002;148(10):2967-73.
- Cepheid. Xpert MTB/XDR [website]. 2021 (https://www.cepheid.com/en/tests/Critical-Infectious-Diseases/Xpert-MTB-XDR).
- Genexpert: negotiated prices [website]. Geneva: Foundation for Innovative New Diagnostics (FIND); 2021 (https://www.finddx.org/pricing/genexpert/).
- Stop TB Partnership. Practical guide to implementing a quality assurance system for Xpert MTB/RIF testing. 2021 (http://www.stoptb.org/wg/gli/pgiqas.asp).
ᵃ Codon numbering system according to Camus et al. (2002) (9), as reported in Cepheid, Clinical evaluation of the Xpert MTBXDR
assay, Report R244C2 Xpert MTB/XDR Rev 1.0.
 Обратная связь
Обратная связь