Перекрёстные ссылки книги для Executive summary
Introduction
Children and young adolescents (aged below 15 years) represent about 11% of all people with tuberculosis (TB) globally. This means that 1.1 million children become ill with TB every year, almost half of them below five years of age. National TB programmes (NTPs) only notify less than half of these children, meaning that there is a large case detection gap (1). The reasons for this gap include challenges with specimen collection and bacteriological confirmation of TB in young children, due to the paucibacillary nature of TB disease in this age group and the lack of highly sensitive point-of-care tests. In 2020, the COVID-19 pandemic had an additional negative impact on TB notifications in children. In addition to the case detection gap, only one third of child contacts below five years of age eligible for TB preventive treatment (TPT) received it in 2020. Young children are at higher risk of developing TB disease, including severe forms of TB, after TB infection, and the majority do so within a few months following exposure and infection (2, 3). In addition to children and young adolescents, over half a million older adolescents (aged 15-19 years) are estimated to develop TB every year (4).
The United Nations Sustainable Development Goal (SDGs) (5) and the World Health Organization (WHO) End TB Strategy (6) include targets to reduce TB incidence by 80% and TB deaths by 90% to be achieved by the year 2030, relative to baseline levels in 2015. In addition, to accelerate progress towards these global targets, the Resolution adopted by the United Nations General Assembly at the High-Level Meeting on the fight against tuberculosis in September 2018 commits to diagnosing and treating 40 million people with TB (including 3.5 million children), and 1.5 million people with drug-resistant TB (DR-TB) (including 115 000 children) by 2022. It also commits to providing at least 30 million people (including 4 million child contacts under five years of age), 20 million other household contacts (including children aged five years and above) and 6 million people living with HIV (including children) with TPT by 2022 (7).
Rationale
To support countries in preventing and managing TB in children and adolescents, WHO's Global Tuberculosis Programme published the WHO Guidance for national tuberculosis programmes on the management of tuberculosis in children (second edition) in 2014 (8). Since the publication of the second edition, new evidence related to diagnostic approaches for TB, treatment for drug-susceptible TB, DR-TB and TB meningitis, as well as models of care relevant to children and adolescents has become available. The WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents (2022) is a consolidated guideline of new and existing recommendations (see annex 1), and replaces the 2014 guidance. It complements existing WHO guidelines on the management of TB, recognizing the unique characteristics and needs of these groups, as well as those of their parents, caregivers and families. The guidelines are complemented by the WHO operational handbook on tuberculosis. Module 5: Management of tuberculosis in children and adolescents, which provides guidance on how to implement the recommendations in the guidelines.
Objectives
The objectives of the 2022 consolidated guidelines are: to provide policy-makers and implementing partners with evidence-based recommendations on the cascade of care for children and adolescents; to support the implementation of activities to prevent TB among children and adolescents at risk; to improve TB case detection and treatment outcomes in children and adolescents with TB using effective models of care; and to contribute to reductions in TB related morbidity and mortality in children and adolescents in line with global targets including those in the SDGs (5), the WHO End TB Strategy (6) and the Political declaration of the UN General Assembly High-Level Meeting on the fight against tuberculosis (7).
Target audience
The target audience for these consolidated guidelines consists primarily of NTPs, primary health care (PHC) programmes, maternal and child health programmes, national AIDS programmes (or their equivalents in health ministries) and other health policy-makers. They also target generalist and specialist paediatricians, clinicians and health practitioners working on TB, HIV and/or infectious diseases in public and private sectors, the educational sector, nongovernmental, civil society and community-based organizations, as well as technical and implementing partners.
Recommendations on the management of TB in children and adolescents
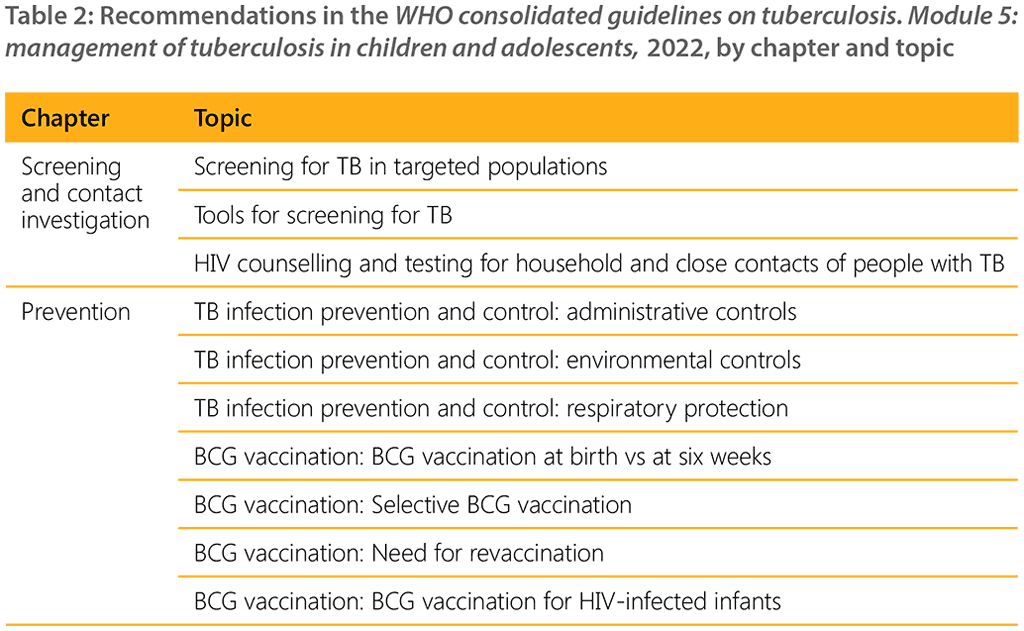
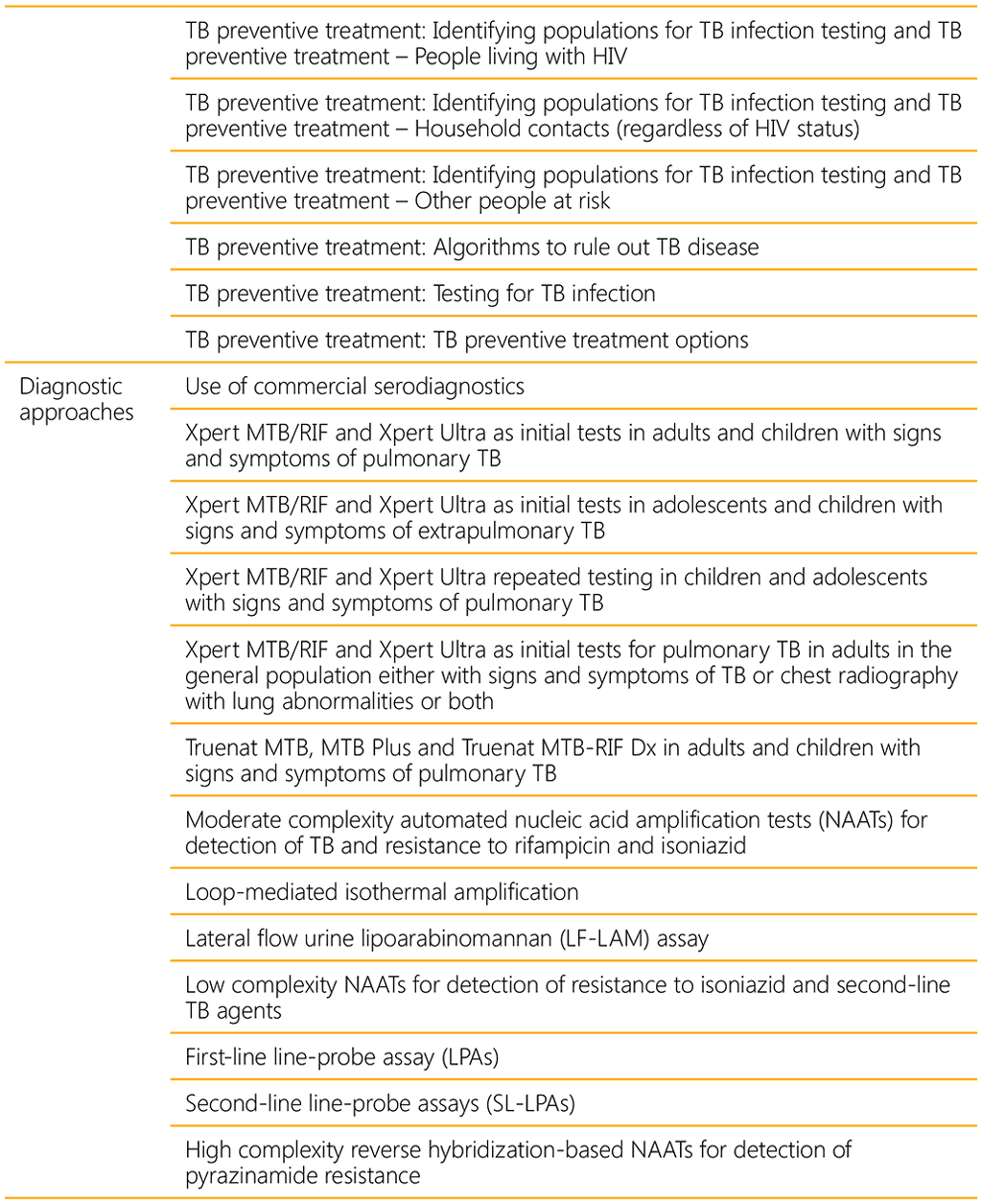
A WHO convened Guideline Development Group (GDG) meeting held in 2021 led to eight new recommendations on the management of TB in children and adolescents (Table 1). A summary of the recommendations consolidated in this guideline are listed in Table 2 below.
A summary of all new and consolidated recommendations can be found in web annex 5.
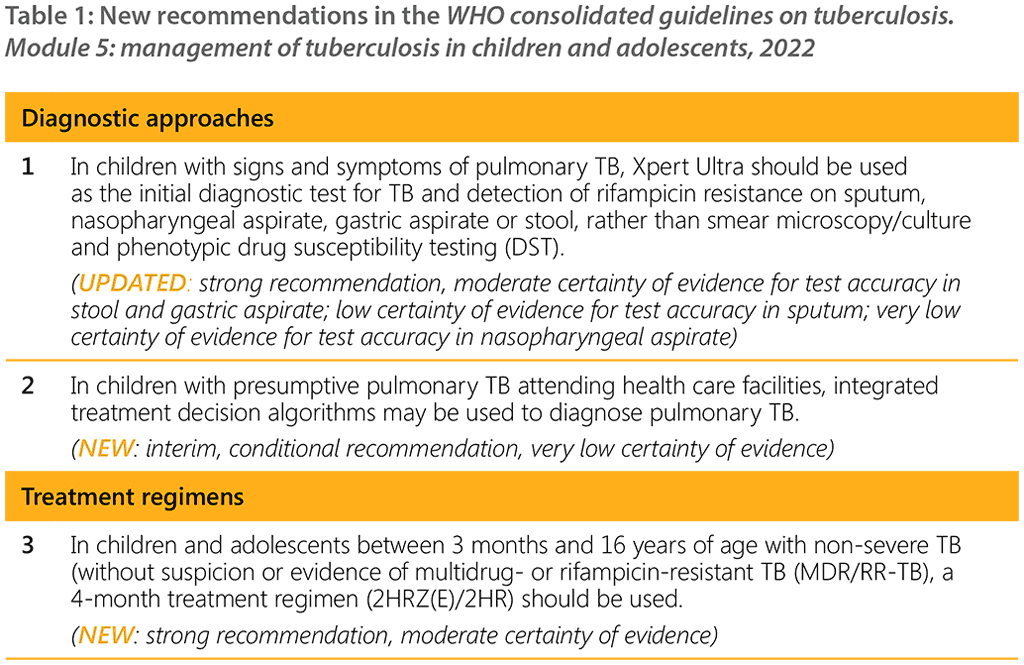
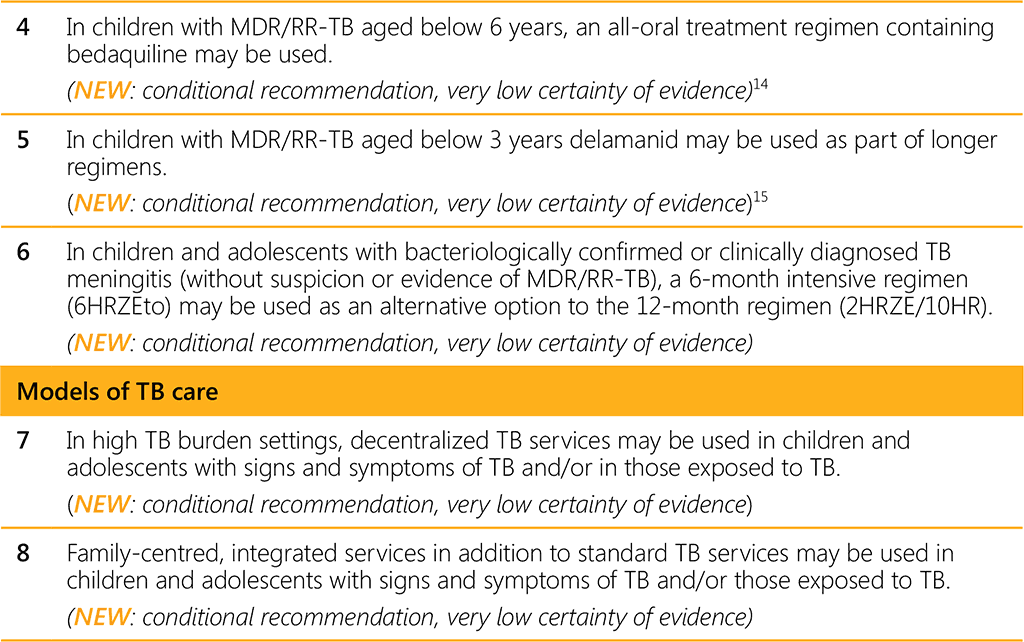
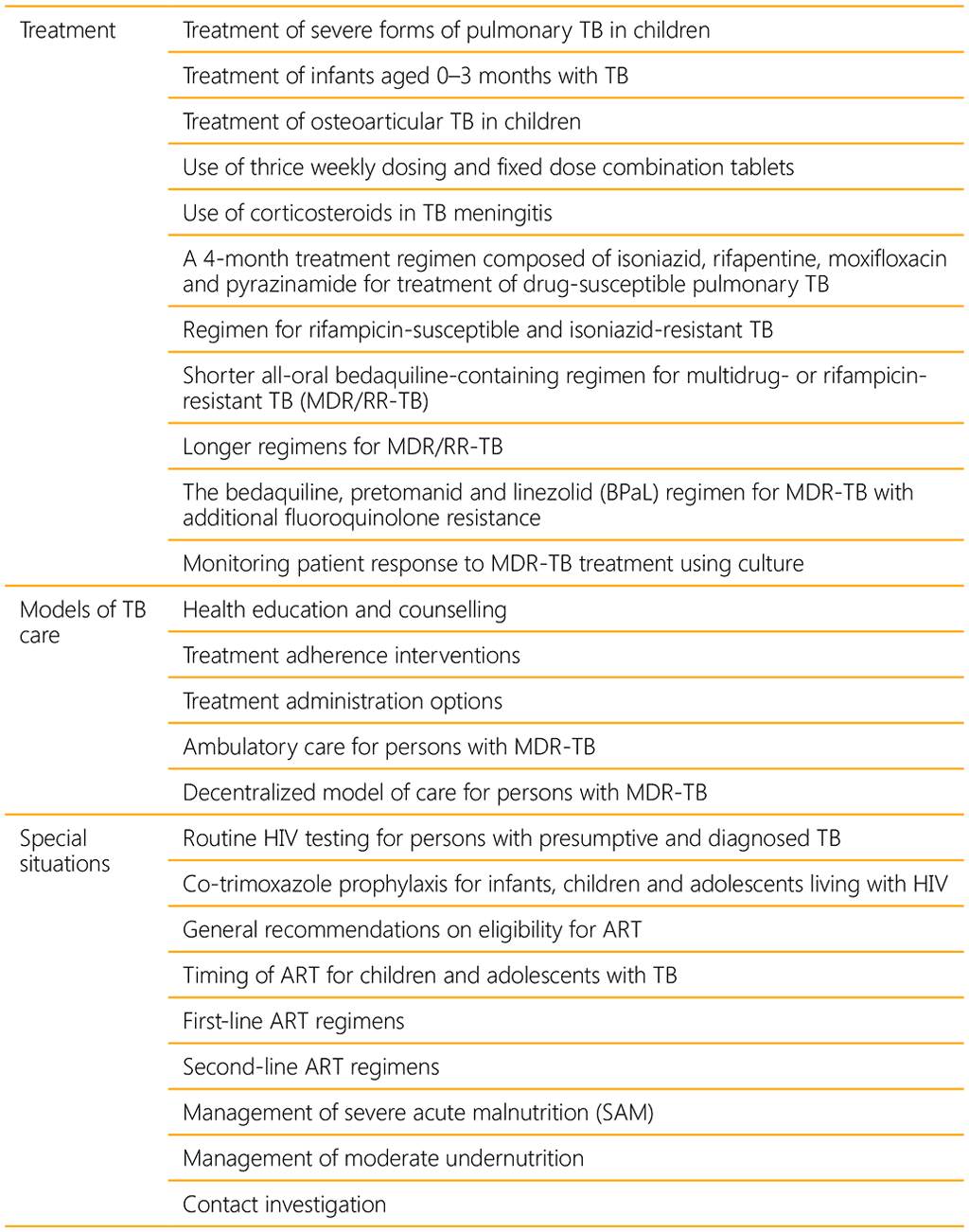
Based on these guidelines, tools to support implementation have been developed, including new integrated treatment decision algorithms and an updated table on dosing of second-line TB medicines. In accordance with the process for updating WHO guidelines, a systematic and continuous process of identifying and bridging evidence gaps following guideline dissemination will be employed. If new evidence that could potentially impact the current evidence base for any of the recommendations is identified, it will be reviewed with a view to updating the recommendation. WHO welcomes suggestions regarding additional questions for inclusion in future updates of the guideline.¹⁶
Main changes to the 2014 guidance in the 2022 update
The 2014 WHO Guidance for national tuberculosis programmes on the management of tuberculosis in children (secondedition) (8) included 28 recommendations on the management of TB in children. The 2022 consolidated guidelines incorporate: recommendations from the 2014 guidelines that remain valid (mainly topics that remain key components of high-quality TB care for which no new evidence was assessed), relevant recommendations that have since been published in other WHO guidelines, and new recommendations published in 2022. A summar y of the changes to the 2014 guidance is provided in the supplementary table in annex 2. Also, the focus of the 2022 consolidated guidelines is on children and adolescents aged 0-19 years, whereas the previous guidelines focused on children and to a lesser extent, younger adolescents (aged 10-14 years).
¹⁴ This recommendation applies to and complements the 2020 WHO recommendations on shorter and longer regimens that contain bedaquiline: A shorter all-oral bedaquiline-containing regimen of 9–12 months duration is recommended in eligible patients with confirmed multidrug- or rifampicin-resistant tuberculosis (MDR/RR-TB) who have not been exposed to treatment with second-line TB medicines used in this regimen for more than one month, and in whom resistance to fluoroquinolones has been excluded (Conditional recommendation, very low certainty in the evidence); bedaquiline should be included in longer multidrug-resistant TB (MDR-TB) regimens for patients aged 18 years or more (Strong recommendation, moderate certainty in the estimates of effect); bedaquiline may also be included in longer MDR-TB regimens for patients aged 6–17 years; (Conditional recommendation, very low certainty in the estimates of effect) (9).
¹⁵ This recommendation complements the 2020 WHO recommendation on longer regimens that contain delamanid: Delamanid may be included in the treatment of MDR/RR-TB patients aged 3 years or more on longer regimens (Conditional recommendation, moderate certainty in the estimates of effect) (9).
¹⁶ The WHO Global Tuberculosis Programme can be contacted at gtbprogramme@who.int.
 Обратная связь
Обратная связь