Перекрёстные ссылки книги для 5.3.2.4. Practical approach to designing individualized multidrug-resistant and rifampicin-resistant TB treatment regimens
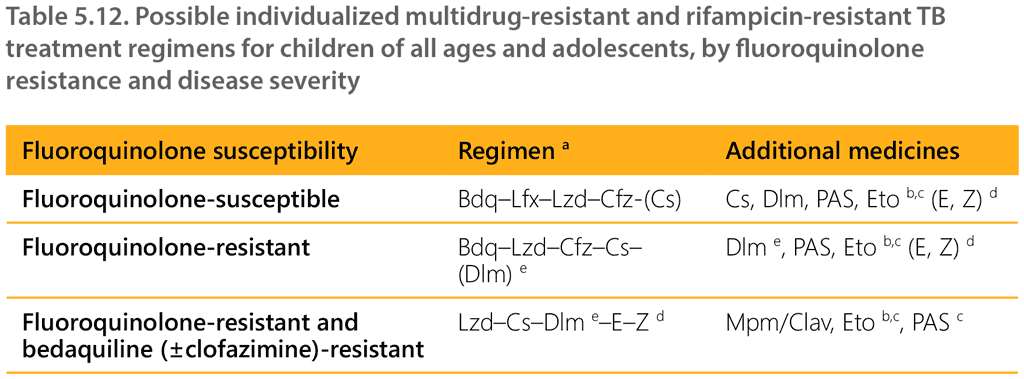
Table 5.12 summarizes possible individualized MDR/RR-TB treatment regimens for children of all ages and adolescents based on the above-described principles and taking into consideration fluoroquinolone and other resistance and eligibility for the shorter regimen.
The bedaquiline, pretomanid and linezolid regimen for multidrug-resistant TB with additional fluoroquinolone resistance in adolescents aged 14 years and over
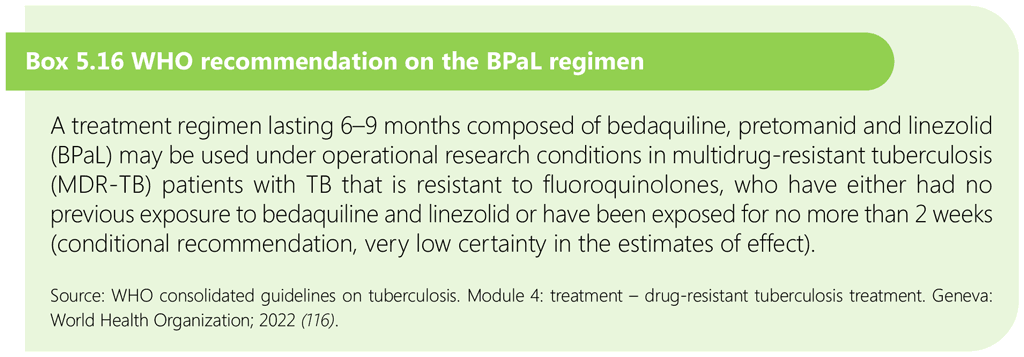
The bedaquiline, pretomanid and linezolid (BPaL) regimen is composed of 6–9 months of bedaquiline, pretomanid and linezolid. It may be used in adolescents aged 14 years and over under operational research conditions conforming to WHO standards, which include research subject to ethical approval, patient-centred care and support, predefined eligibility criteria, patient-informed consent, implementation according to the principles of good clinical practice, active drug safety monitoring and management, treatment monitoring, outcome evaluation, and comprehensive standardized data collection.
Eligibility criteria for treatment with the BPaL regimen are all of the following:
- bacteriologically confirmed PTB and laboratory-confirmed resistance to rifampicin and fluoroquinolones with or without resistance to injectable agents;
- age at least 14 years at the time of enrolment;
- weight 35 kg or over;
- informed consent to be enrolled in the operational research project and to adhere to the follow-up schedule (signed or witnessed consent if the patient is illiterate, or signed or witnessed consent from a child’s parent or legal guardian);
- for adolescent girls, no pregnancy or breastfeeding and willingness to use effective contraception;
- no known allergy to any BPaL component medicines;
- no evidence in DST results of resistance to any of the component medicines, or no previous exposure to any of the component medicines for 2 weeks or more;
- no EPTB, including meningitis, other CNS TB or TB osteomyelitis.
Further details on the BPaL regimen can be found in the WHO operational handbook on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment (82)
 Обратная связь
Обратная связь