Liens transversaux de livre pour 10.2 Considerations for implementation
It is both important and feasible for NTPs to ascertain cure at the end of treatment. The notion of relapse-free cure or sustained treatment success after the end of treatment is critical; however, it is beyond the means of routine programmatic monitoring and is feasible only under operational research conditions (e.g. in special cohorts, in patients undergoing rehabilitation and during follow-up for post-TB lung disease). For this reason, the specific operational definition “sustained treatment success” was proposed (Table 10.1), with the possibility of assessing numbers of patients alive and free of TB at 6 months (for DS-TB and DR-TB) and at 12 months (for DR-TB only) after successful TB treatment
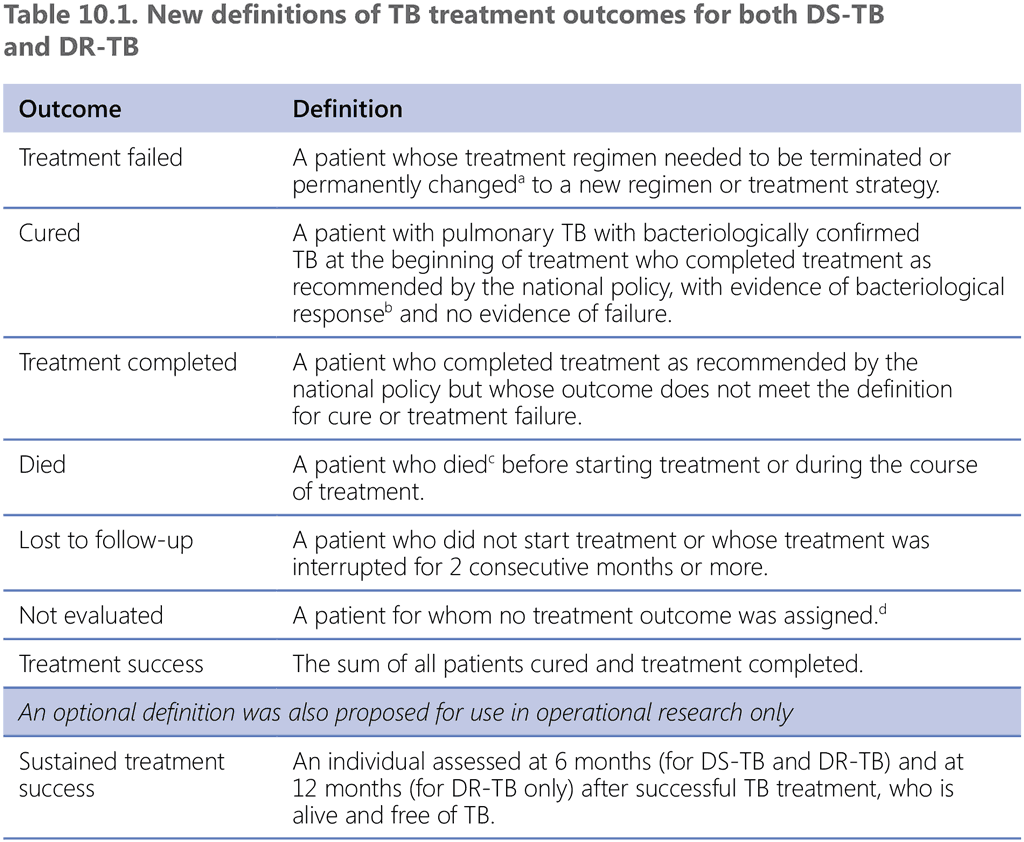
DR-TB: drug-resistant TB; DS-TB: drug-susceptible TB; TB: tuberculosis.
ᵃ Reasons for the change include:
- no clinical response or no bacteriological response, or both (see note ‘b’);
- adverse drug reactions; or
- evidence of additional drug-resistance to medicines in the regimen.
ᵇ “Bacteriological response” refers to bacteriological conversion with no reversion:
- “bacteriological conversion” describes a situation in a patient with bacteriologically confirmed TB where at least two consecutive cultures (for DR-TB and DS-TB) or smears (for DS-TB only) taken on different occasions at least 7 days apart are negative; and
- “bacteriological reversion” describes a situation where at least two consecutive cultures (for DR-TB and DS-TB) or smears (for DS-TB only) taken on different occasions at least 7 days apart are positive either after the bacteriological conversion or in patients without bacteriological confirmation of TB.
ᶜ Patient died for any reason.
ᵈ This includes cases “transferred out” to another treatment unit and whose treatment outcome is unknown; however, it excludes those lost to follow-up.
 Retour
Retour