Liens transversaux de livre pour 4.1 Algorithm 1 – mWRD as the initial diagnostic test for TB
Algorithm 1 is the preferred algorithm for testing to support the diagnosis of TB in individuals being evaluated for pulmonary and extrapulmonary TB, and to achieve universal DST. In this algorithm, mWRDs are used as the initial diagnostic test to detect TB, RIF resistance (except if TB-LAMP is used) and – in the case of using moderate complexity automated NAATs – INH resistance (i.e. this algorithm meets the goals of the End TB Strategy for the use of mWRDs and universal DST). If TB-LAMP is used, follow-on testing for RIF resistance is needed. This algorithm is designed to be used with any of the mWRDs for detection of MTBC (Xpert MTB/RIF, Xpert Ultra, Truenat MTB, Truenat MTB Plus and TB-LAMP, moderate complexity automated NAAT), although the algorithm may need to be modified, depending on which mWRD is used and in which population. For example, in a setting with a high MDR-TB burden, it would be preferable to use an mWRD that detects MTBC and RIF resistance initially (e.g. Xpert MTB/RIF, Truenat MTB then Truenat MTB-RIF Dx or moderate complexity automated NAAT) rather than one that detects only MTBC (e.g. TB-LAMP). In a setting with a well-functioning referral system and a high risk of Hr-TB, a moderate complexity automated NAAT may be preferred as the initial test because of the ability to test for INH and RIF resistance simultaneously.
This algorithm is feasible when the mWRD testing can be conducted on site or can be accessed through a reliable referral system with short turnaround times.
Fig. 4.2. Algorithm 1: Molecular WRD as the initial diagnostic test for TB
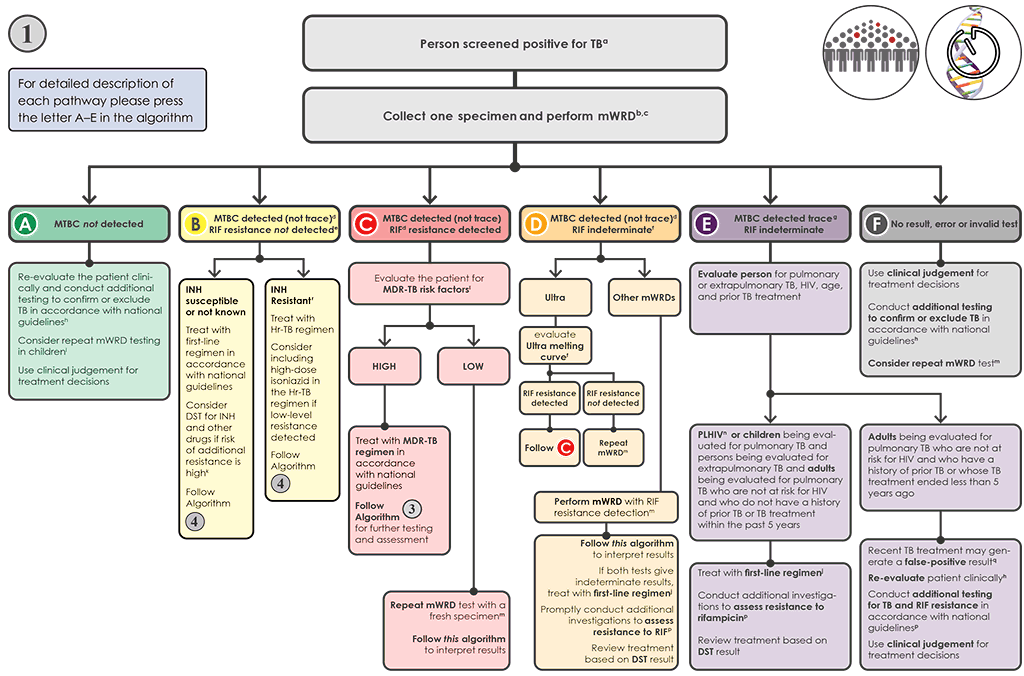
AIDS: acquired immunodeficiency syndrome; CSF: cerebrospinal fluid; DNA: deoxyribonucleic acid; DST: drug susceptibility testing; EMB: ethambutol; HIV: human immunodeficiency virus; HREZ: isoniazid (H), rifampicin (R), ethambutol (E) and pyrazinamide; Hr-TB: isoniazid-resistant, rifampicin-susceptible TB; INH: isoniazid; LAMP: loop-mediated isothermal amplification; LPA: line probe assay; MC-aNAAT: moderate complexity automated nucleic acid amplification test; MDR-TB: multidrug-resistant TB; MTB: Mycobacterium tuberculosis; MTBC: Mycobacterium tuberculosis complex; mWRD: molecular WHO-recommended rapid diagnostic test; PLHIV: people living with HIV/AIDS; PZA: pyrazinamide; RIF: rifampicin; RR-TB: rifampicin-resistant TB; TB: tuberculosis; WHO: World Health Organization; WRD: WHO-recommended rapid diagnostic test.
a People screened positive for TB include adults and children with signs or symptoms suggestive of TB, with a chest X-ray showing abnormalities suggestive of TB, a positive mWRD used as a screening tool or positive C-reactive protein test (>5 mg/L) in PLHIV. A person with a positive mWRD used as a screening tool and a low pretest probability should be clinically assessed and, if deemed to be a person presumed to have TB, should have a repeat mWRD performed and follow Algorithm 1. If the pretest probability is high and the clinical picture is consistent with TB disease, then this test could be considered diagnostic and the person should be managed based on the result of the test and, if relevant, should continue on to Algorithm 3 or 4. This algorithm may also be followed for the diagnosis of extrapulmonary TB using CSF, lymph node and other tissue specimens. However, mWRDs that are recommended for use in the diagnosis of extrapulmonary TB investigations are currently limited to Xpert MTB/RIF and Xpert Ultra.
b Programmes may consider collecting two specimens upfront. The first specimen should be promptly tested using the mWRD. The second specimen may be used for the additional testing described in this algorithm. For individuals being evaluated for pulmonary TB, sputum is the preferred specimen. Tissue biopsy samples are difficult or impossible to obtain repeatedly; therefore, they should be tested with as many methods as possible (e.g. mWRD, culture, DST or histology).
c mWRDs or classes appropriate for this algorithm include Xpert MTB/RIF, Xpert Ultra, Truenat MTB, Truenat MTB Plus, MC-aNAAT and TB-LAMP.
d “MTBC detected (not trace)” includes MTBC detected as high, medium, low or very low. These categories apply to the Xpert MTB/RIF and Xpert Ultra tests. Results of the Truenat MTB and MTB Plus tests, MC-aNAAT and the TB-LAMP test also fall into the category of “MTBC detected (not trace)”. The MC-aNAAT provides additional resistance detection for isoniazid and leads to additional considerations in Box B.
e Determination of RIF resistance occurs simultaneously in the Xpert MTB/RIF, Xpert Ultra and MC-aNAAT tests. A second test is needed to determine RIF resistance in the Truenat MTB or MTB Plus test, using the same DNA isolated for the Truenat MTB tests (Truenat MTB-RIF Dx test) and in the TB-LAMP test, which requires a fresh specimen to be collected and a molecular or phenotypic DST to be conducted. In the case of MC-aNAAT, INH resistance detection would also occur simultaneously with RIF detection.
f The interpretation and follow-up testing for MTBC detected and RIF indeterminate or unknown for the Xpert Ultra test differs from the interpretation of results for other mWRDs. MTBC detected that RIF indeterminate results obtained with the Xpert Ultra test (especially those with high and medium semiquantitative results) may be due to large deletions or multiple mutations that confer RIF resistance. Analysis of the Ultra melt curves can detect such resistance-conferring mutations. In some cases, culture and DST, sequencing or alternative mWRD will be needed to confirm or exclude RIF resistance. Indeterminate results for the other mWRDs are usually related to very low numbers of bacilli in the sample. When using an mWRD test without detection of RIF resistance (e.g. TB-LAMP), further testing for RIF resistance is required using an mWRD able to detect resistance.
g “MTBC detected trace” applies only to the Xpert Ultra test.
h Further investigations for TB may include chest X-ray, additional clinical assessments, repeat mWRD testing, culture or clinical response following treatment with broad-spectrum antimicrobialagents.
i In children with signs and symptoms of pulmonary TB in settings with a pretest probability of 5% or more, and an Xpert MTB/RIF or Xpert Ultra negative result on the initial test, repeat testing with Xpert MTB/RIF or Ultra (for a total of two tests) in sputum or nasopharyngeal aspirate. Furthermore, repeated testing with Xpert MTB/RIF may be used only in gastric fluid, and stool specimens. No data were available to assess the performance of Xpert Ultra in gastric fluid and stool specimens. Programmes are encouraged to use Xpert Ultra in gastric fluid and stool specimens under operational research conditions. The mWRD should be repeated at the same testing site with a fresh specimen, with the result of the repeat test interpreted as shown in this algorithm. The result of the second test is the result that should be used for clinical decisions.
j People should be initiated on a first-line regimen according to national guidelines, unless the person is at very high risk of having MDR-TB. Such people should be further investigated and initiated on an MDR-TB regimen. In situations where INH results are available (e.g. MC-aNAAT) and INH resistance has not been detected, the probability of having MDR-TB would be lower.
k A sample may be sent for molecular or phenotypic DST if there is a high prevalence of INH or other drug resistance and RIF susceptible (i.e. INH mono- or poly-resistance) in this setting. Where a result for INH resistance is “not detected” (e.g. MC-aNAAT), and the pretest probability for Hr-TB is high, phenotypic DST for INH should be performed.
l People at high risk for MDR-TB include people who were treated previously, including those who had been lost to follow-up, relapsed or experienced treatment failure; non-converters (smear- positive at end of intensive phase); MDR-TB contacts; and any other groups at risk for MDR-TB identified in the country.
m The mWRD with RIF testing should be performed at the same testing site with a fresh specimen, and the result of the second test should be interpreted as shown in this algorithm. The RIF result from the second test is the result that should be used for clinical decisions.
n PLHIV include those who are HIV-positive or whose HIV status is unknown, but who present with strong clinical evidence of HIV infection, reside in settings where there is a high prevalence of HIV or are members of a group at risk for HIV. For all those with unknown HIV status, HIV testing should be performed according to national guidelines.
o People should be promptly initiated on an MDR-TB regimen in accordance with national guidelines. Algorithm 3 should be followed for additional testing for any person with RR-TB.
p Phenotypic (culture and DST) and molecular (e.g. alternate mWRDs, LPAs and targeted NGS) methods are available for evaluating drug resistance. Rapid molecular methods are preferred.
q In people with a prior history of TB within the past 5 years or whose TB treatment was completed less than 5 years ago, Xpert Ultra trace results (and occasionally Xpert MTB/RIF “MTBC detected low or very low”) may be positive, not because of active TB but because of the presence of non-viable bacilli. Clinical decisions must be made based on all available information and clinical judgement.
r People diagnosed using an MC-aNAAT and whose result is RIF resistance not detected and INH resistance detected should be treated for Hr-TB with RIF/EMB/PZA (REZ) and levofloxacin. For practical purposes, HREZ fixed-dose combination tablets may be used instead of REZ. Consider including high-dose INH in the Hr-TB regimen if low-level resistance is detected (inhA mutation only). Follow Algorithm 4.
 Retour
Retour