Liens transversaux de livre pour 5.4.1 DST results
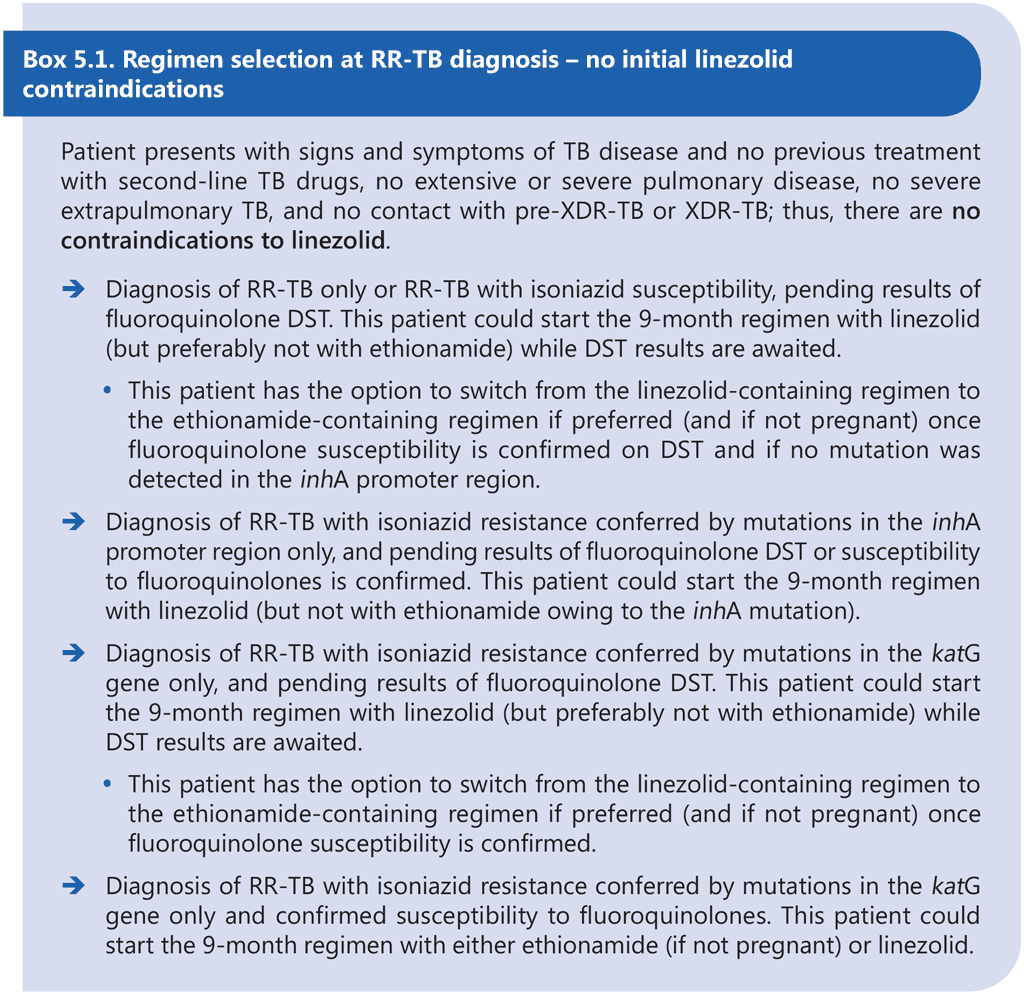
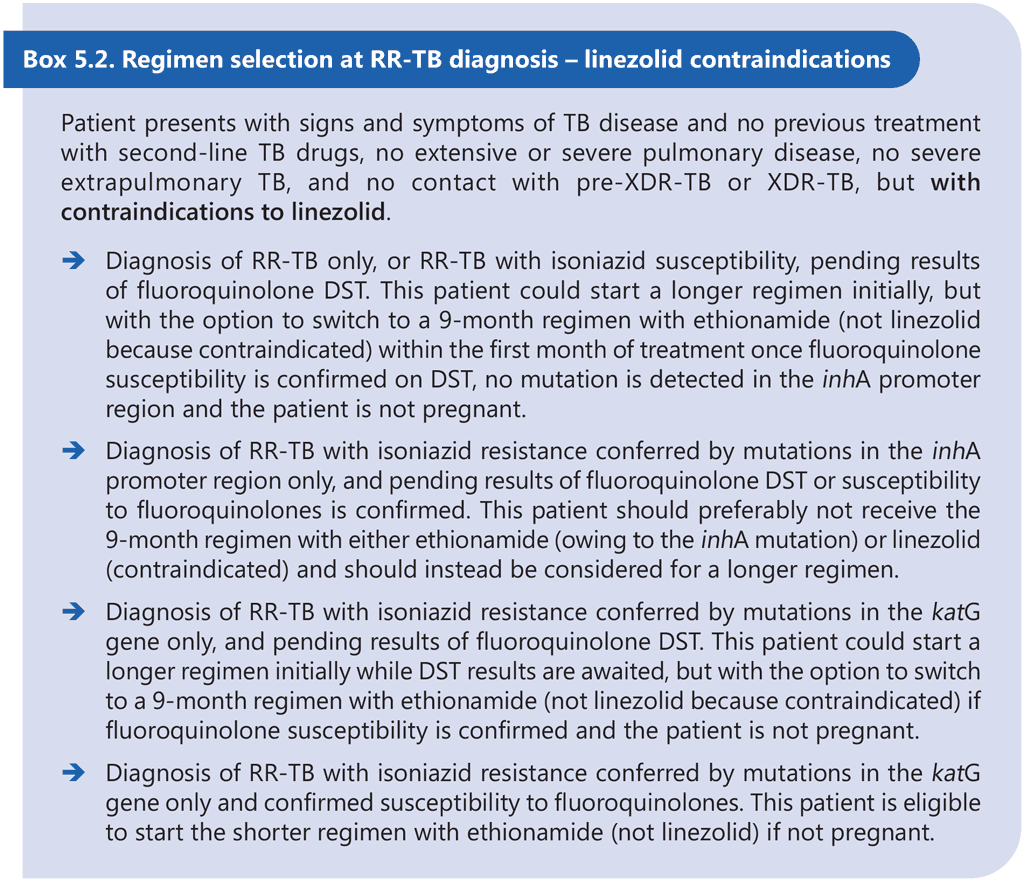
The 9-month all-oral regimen is not adequate for the treatment of pre-XDR-TB or XDR-TB, and the efficacy of the regimen for treatment of MDR-TB where both inhA and katG mutations are present is largely unknown. Therefore, DST is recommended at or before the start of this regimen to exclude resistance to at least fluoroquinolones and to determine the mutations conferring resistance to isoniazid. Results of DST should not delay the start of an appropriate MDR/RR-TB treatment regimen, particularly if DST relies on phenotypic methods. Although there appears to be no significant association between treatment delay and individual MDR/RR-TB treatment outcomes, delays in effective treatment initiation increase the risk of clinical deterioration and contribute to ongoing transmission of MDR/RR-TB (51, 52). Provided that there is no history or evidence of exposure to fluoroquinolone-resistant MDR/ RR-TB, the 9-month regimen containing linezolid (instead of ethionamide) may be started in eligible patients with TB that is resistant to at least rifampicin while awaiting fluoroquinolone DST results. If a linezolid-containing 9-month regimen cannot be offered while awaiting fluoroquinolone DST results (e.g. owing to contraindications to linezolid), then the patient may start an effective longer regimen with the option to switch to an ethionamide-containing 9-month regimen if fluoroquinolone resistance is definitively ruled out once DST results become available within the first month of treatment and all other eligibility criteria are met. Linezolid may offer some initial protection against as-yet-undetected fluoroquinolone resistance at the start of treatment, but ethionamide probably does not offer the same level of protection; therefore, the 9-month regimen containing ethionamide should only be considered if results of fluoroquinolone DST are available and indicate susceptibility to fluoroquinolones prior to initiation of this regimen. Box 5.1 and Box 5.2 below provide examples of clinical scenarios and appropriate choices of regimens.
The LPA, MTBDRplus, is widely used to detect mutations conferring resistance to isoniazid, as well as rifampicin; however, in future this test may be replaced by the Xpert MTB-XDR assay cartridge in some settings. Using the GeneXpert® platform, this assay detects mutations associated with resistance to isoniazid, fluoroquinolones, second-line injectable drugs and ethionamide in a single test. The result for isoniazid resistance is reported as “high-level” or “low-level” on this platform, and additional laboratory expertise may be required to identify the specific mutations conferring resistance to isoniazid and ethionamide. In general, RR-TB isolates reported to have “high-level” isoniazid resistance as well as ethionamide resistance could be assumed to have both inhA and katG mutations, and the 9-month regimen may not be appropriate for treatment in these cases. Molecular testing methods have a lower sensitivity for detecting isoniazid resistance than phenotypic methods; hence, in the presence of RR-TB, isoniazid susceptibility demonstrated on genotypic testing must be confirmed phenotypically before a normal dose of isoniazid is used within the 9-month all-oral regimen.
DST for pyrazinamide and ethambutol is not carried out routinely in most settings; therefore, this information is rarely available to guide treatment decisions at an individual patient level. Phenotypic DST for these drugs is often included in regional or nationwide drug-resistance surveys and this information might be useful when deciding whether to use the 9-month regimen including these drugs. The 9-month all-oral regimen is standardized and all seven drugs (including either ethionamide or linezolid) should be initiated from the outset. Pyrazinamide and ethambutol are inexpensive and generally well tolerated, and may still be efficacious against MDR/RR-TB in people who are eligible for this regimen. However, some health care providers and patients, particularly children and their caregivers, might find the high pill burden posed by these medicines challenging despite the shorter duration of this treatment regimen.
Ideally, phenotypic DST for clofazimine, linezolid and bedaquiline should be performed at the time of treatment initiation or with the first strain isolated from patients’ samples during treatment monitoring. TB programmes must rapidly build the capacity to undertake DST, and all efforts must be made to ensure access to approved tests. If routine phenotypic DST for clofazimine, linezolid and bedaquiline is not feasible for all patients diagnosed with MDR/RR-TB, then DST for these drugs must be prioritized for patients with positive TB sputum cultures at month 4 of treatment or beyond. Delayed conversion to negative cultures, or reversion to positive cultures, after 4 months of MDR/RR-TB treatment may be an early indication that treatment is failing, and clinicians must consider the possibility of acquired drug resistance (53).
 Retour
Retour