Liens transversaux de livre pour 3.2 Pretest probability and test accuracy considerations
The predictive values of a test vary depending on the prevalence of TB in the population being tested. Table 3.1 provides examples of population-level projections of the results of testing with the various mWRDs in settings with different levels of TB prevalence, based on pooled sensitivity and specificity estimates that were extracted from the WHO consolidated guidelines on tuberculosis. Module 3: Diagnosis – rapid diagnostics for tuberculosis detection, 2021 update (13) for each test. Tables 3.2–3.4 provide those same parameters for detection of resistance to RIF, INH and FQs, respectively. Tables 3.5–3.6 provide those same parameters for detection of resistance to first-line and second-line anti-TB agents using targeted NGS tests. The sensitivity of the test may be lower when used for active case finding in a population screening context because such people would be less ill and have a lower bacillary burden. In choosing a test to implement, countries will need to consider the possible trade-offs between higher or lower sensitivity and higher or lower specificity, based on the prevalence of TB in their country. False negative results may lead to missed opportunities to treat TB. False positive results may lead to the overtreatment of people who do not have TB. In some settings, countries may need to conduct additional modelling work to support decisions on implementation strategies, based on the trade-offs between sensitivity and specificity in their settings.
Usually, a decision to undertake a diagnostic work-up of an individual for TB begins with assessing symptoms and signs of TB disease. However, many individuals with culture-positive TB may not have symptoms or may consider the symptoms too insignificant to report, leading to missed diagnostic opportunities. To improve TB case detection and identify individuals suitable for TB preventive treatment, WHO has updated the TB screening guidelines (33). Several modalities are recommended for screening: symptom screening, chest X-ray and mWRDs. For screening PLHIV, recommended screening modalities include the classical four-symptom screen, chest X-ray, and an mWRD or positive C-reactive protein test (>5 mg/L).
Chest X-ray as a screening or triage tool can identify individuals to be tested with an initial molecular test. Thus, it can reduce the number of individuals tested and the associated costs, but the cost of the radiography would need to be lower than the cost of the test (33). This approach is likely to improve the pretest probability for TB; therefore, it should improve the predictive value of the molecular test and reduce false positive results, especially in populations with a low prevalence of TB. For example, the addition of chest X-ray as a screening tool to an algorithm in which all individuals with an abnormal chest X-ray receive mWRD was calculated to increase the positive predictive value of the mWRD from 56.8% to 78.5%, and the prevalent cases detected from 69% to 80%, compared with testing with an mWRD, irrespective of symptoms, in a population with a TB prevalence of 1% (33).
Table 3.1. Performance of mWRDs for the detection of TB in adults with signs and symptoms being evaluated for pulmonary TB compared with a microbiological reference standard (absolute number of TP, FP, TN or FN, under a given prevalence out of 1000)
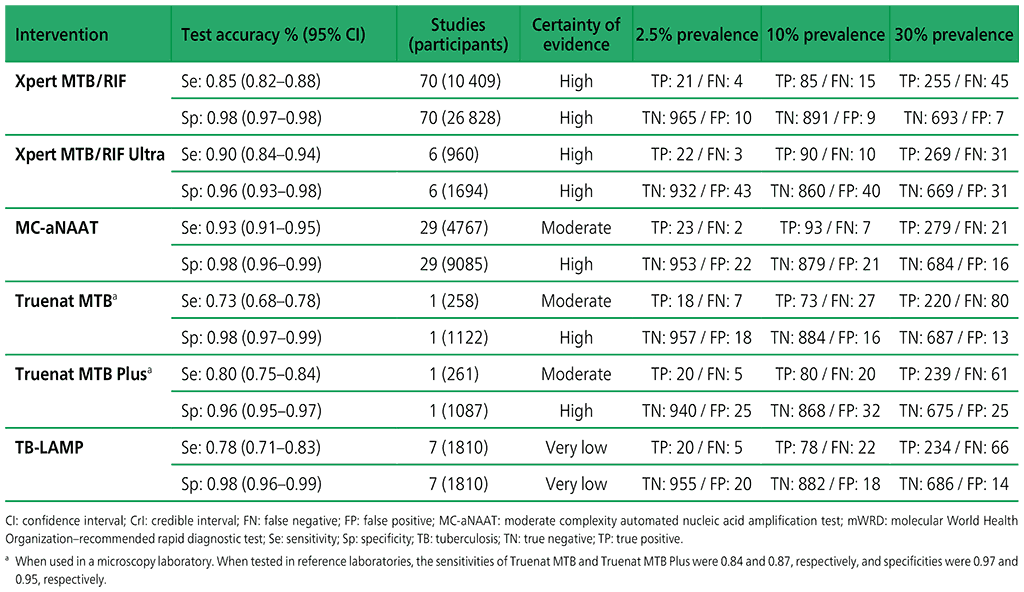
Table 3.2. Performance of molecular tests for the detection of rifampicin resistance in adults with signs and symptoms being evaluated for pulmonary TBa compared with a microbiological reference standard (absolute number of TP, FP, TN or FN, under a given prevalence of rifampicin resistance out of 1000)
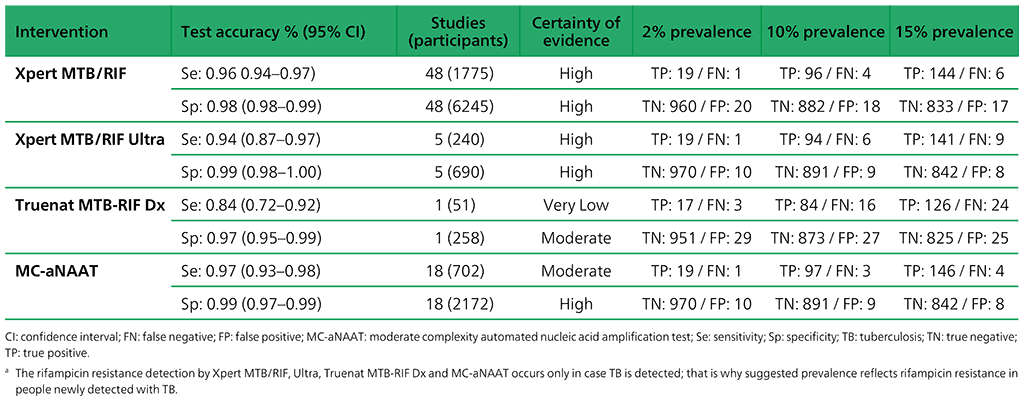
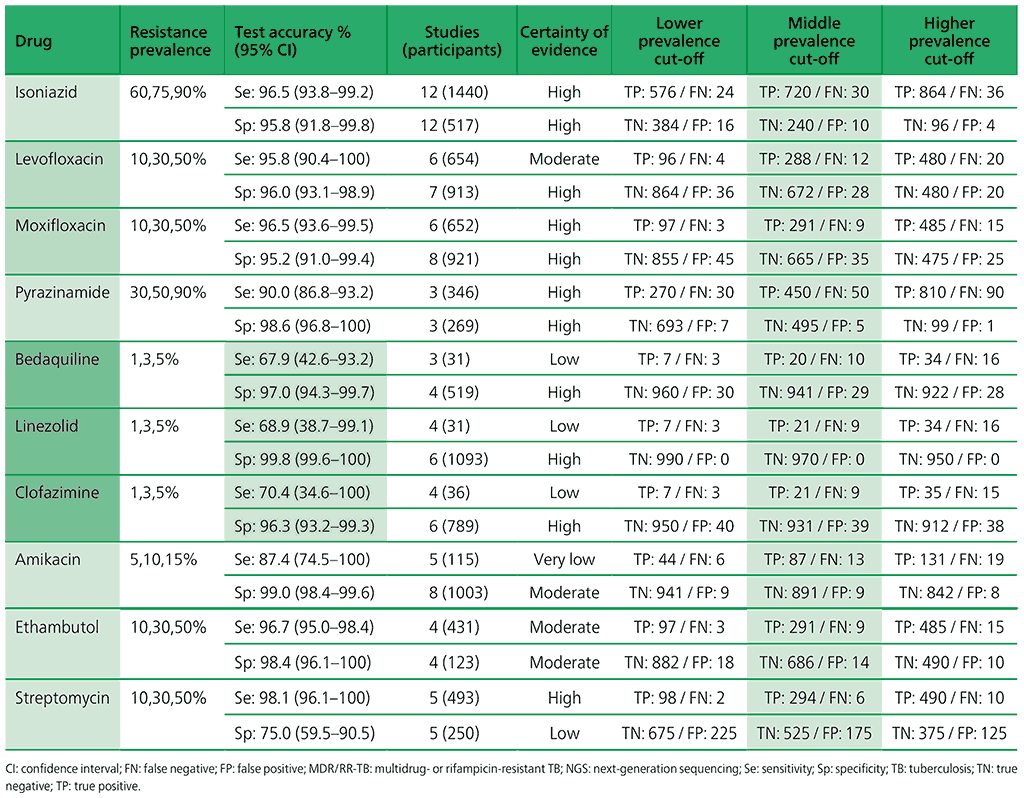
Table 3.3. Performance of molecular tests for the detection of isoniazid resistance in adults with detected pulmonary TBa compared with a microbiological reference standard (absolute number of TP, FP, TN or FN, under a given prevalence of isoniazid resistance out of 1000)
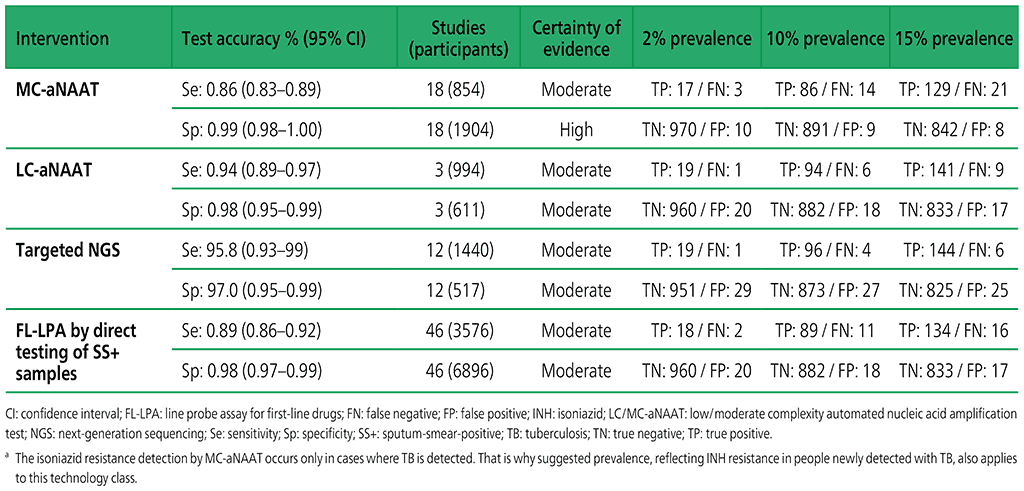
Table 3.4. Performance of molecular tests for the detection of fluoroquinolone resistance in adults with detected pulmonary TB compared with a microbiological reference standard (absolute number of TP, FP, TN or FN, under a given prevalence of fluoroquinolone resistance out of 1000)
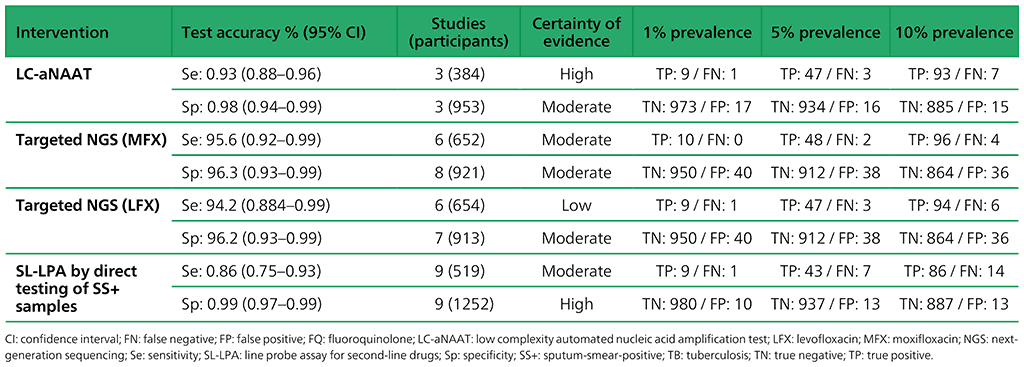
Table 3.5. Performance of molecular tests for the detection of resistance to other first-line anti-TB medicines in adults with detected pulmonary TB compared with a microbiological reference standard (absolute number of TP, FP, TN or FN, under a given prevalence of resistance out of 1000)
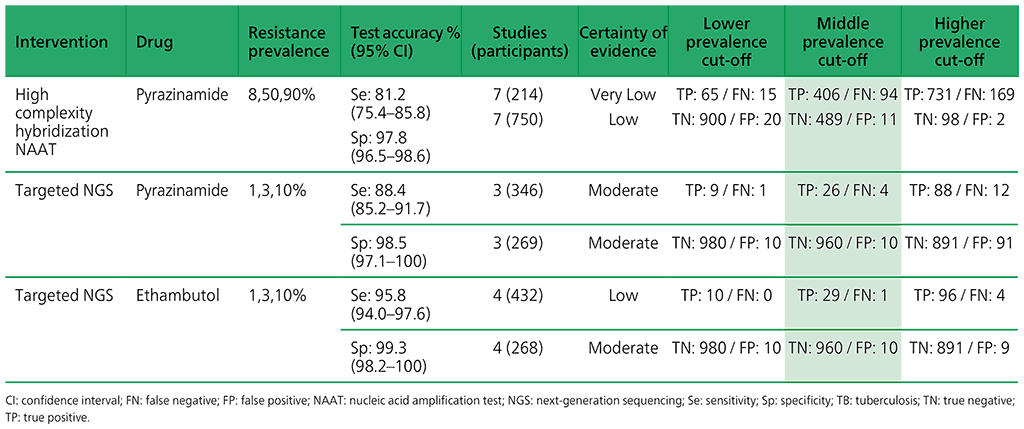
Table 3.6. Performance of targeted NGS for the detection of resistance to anti-TB medicines used to treat MDR/RR-TB in adults with bacteriologically confirmed rifampicin-resistant pulmonary TB compared with a microbiological reference standard (absolute number of TP, FP, TN or FN, under a given prevalence of resistance out of 1000)
 Retour
Retour