Liens transversaux de livre pour Executive summary
Purpose of the guideline¹
Undernutrition increases the risk of tuberculosis (TB) and in turn TB can lead to malnutrition. Undernutrition is therefore highly prevalent among people with TB. It has been demonstrated that undernutrition is a risk factor for progression from TB infection to active TB disease and that undernutrition at the time of diagnosis of active TB is a predictor of increased risk of death and TB relapse. However, the evidence concerning the effect of nutritional supplementation on TB prevention and health outcomes among people with TB had not previously been systematically reviewed. This guideline provides guidance on the principles and recommendations for nutritional care and support of patients with TB as part of their regular TB care. However, it does not consider the provision of food as part of a package of enablers to improve TB treatment adherence or as means to mitigate the negative financial consequences of TB. Member States have requested guidance from the World Health Organization (WHO) on nutritional care and support for patients with TB, in support of their efforts to achieve the Millennium Development Goals. The primary audience for the guideline is health workers providing care to people with TB. However, the guideline is also intended for a wider audience, including policy-makers, their expert advisers, and technical and programme staff at organizations involved in the design, implementation and scaling-up of nutrition actions for public health.
Guideline development methodology
WHO developed the present evidence-informed recommendations using the procedures outlined in the WHO handbook for guideline development (1). The steps in this process included: (i) identification of priority questions and outcomes; (ii) retrieval of the evidence; (iii) assessment and synthesis of the evidence; (iv) formulation of recommendations, including research priorities; and (v) planning for dissemination, implementation, impact evaluation and updating of the guideline. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was followed, to prepare evidence profiles related to preselected topics, based on upto-date systematic reviews.
The guideline development group for nutrition interventions, the Nutrition Guidance Advisory Group, comprised content experts, methodologists, representatives of potential stakeholders and consumers. These experts participated in three WHO technical consultations concerning this guideline, held in 2009–2011, two in Geneva, Switzerland, and one in Amman, Jordan. Members of the external experts’ and stakeholders’ panel were identified through a public call for comments, and this panel was involved throughout the guideline development process.
Available evidence
Three systematic reviews were updated to inform this process. One Cochrane systematic review assessed the effects of oral nutritional supplements (food, protein/energy supplements or micronutrients) on TB treatment outcomes and nutritional recovery in people on anti-tuberculous drug therapy for active TB. The review included 23 trials and concluded that there is insufficient research to determine whether the routine provision of food or energy supplements results in better TB treatment outcomes, or improved quality of life. Although blood levels of some vitamins may be low in patients starting treatment for active TB, there is currently no reliable evidence that routine micronutrient supplementation at or above recommended daily amounts has clinical benefits. Another systematic review investigated the optimal composition of diet for patients receiving treatment for active TB. This review included only two studies, both on energy requirements in people on TB treatment. The review concluded that the evidence is too weak to judge whether, or by how much, the daily energy intake needs are increased in people with active TB. A third review assessed the effects of nutritional interventions on the progression from TB infection to active TB. No trial was included in the review. There is no evidence on reduced risk of progression because of nutrient supplementation.
The overall evidence base on effects of nutritional supplements for TB prevention and care is limited and the overall quality is low or very low for most outcomes. There is no evidence on improvement of TB treatment outcomes, or prevention of progression from TB infection to active disease, when using nutritional supplementation as an addition to standard care.
Principles
Five guiding principles¹ are key for providing nutritional care and support as an integral part of TB care and prevention.
1. All people with active TB should receive TB diagnosis, treatment and care according to WHO guidelines and international standards of care. When malnutrition is identified at the time of TB diagnosis, TB is considered a key causal factor that needs to be addressed. It is essential that nutrition assessment and assistance do not divert resources from optimal TB diagnosis and care. Concerns about weight loss or failure to gain weight during TB treatment should trigger further clinical assessment (e.g. resistance to TB drugs, poor adherence, comorbid conditions) and nutrition assessment of the causes of undernutrition, in order to determine the most appropriate interventions.
2. An adequate diet, containing all essential macro- and micronutrients, is necessary for the well-being and health of all people, including those with TB infection or TB disease.
3. Because of the clear bidirectional causal link between undernutrition and active TB, nutrition screening, assessment and management are integral components of TB treatment and care.
4. Poverty and food insecurity are both causes and consequences of TB, and those involved in TB care therefore play an important role in recognizing and addressing these wider socioeconomic issues.
5. TB is commonly accompanied by comorbidities such as HIV, diabetes mellitus, smoking and alcohol or substance abuse, which have their own nutritional implications, and these should be fully considered during nutrition screening, assessment and counselling.
Recommendations
Patients with TB should be nutritionally assessed and receive the same nutritional care and support as other individuals or populations of similar nutritional status, in agreement with all relevant WHO recommendations.
The recommendations are grouped on four areas related to nutritional care and support, to cover especially vulnerable populations, with an additional area for contact investigation.
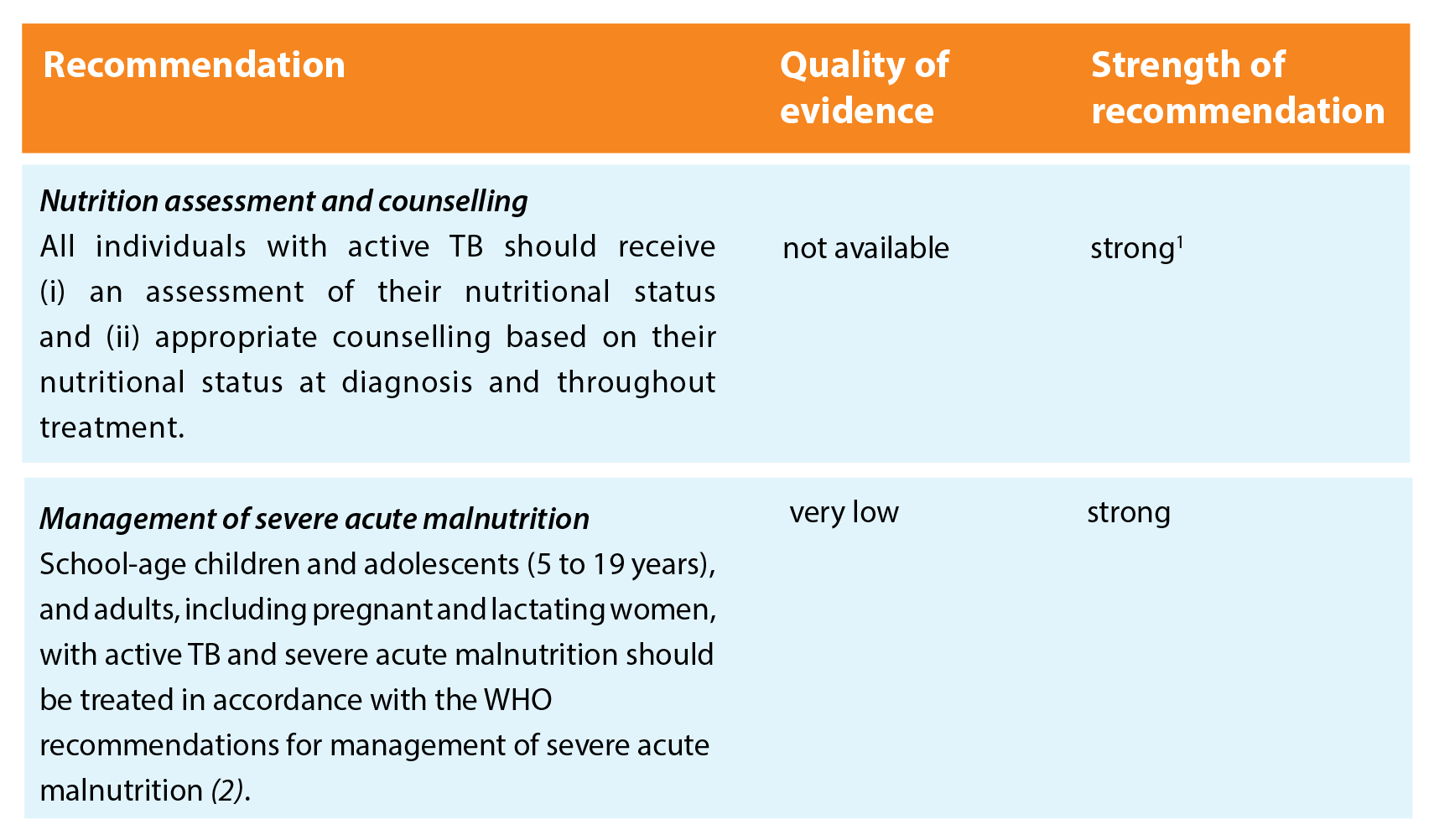
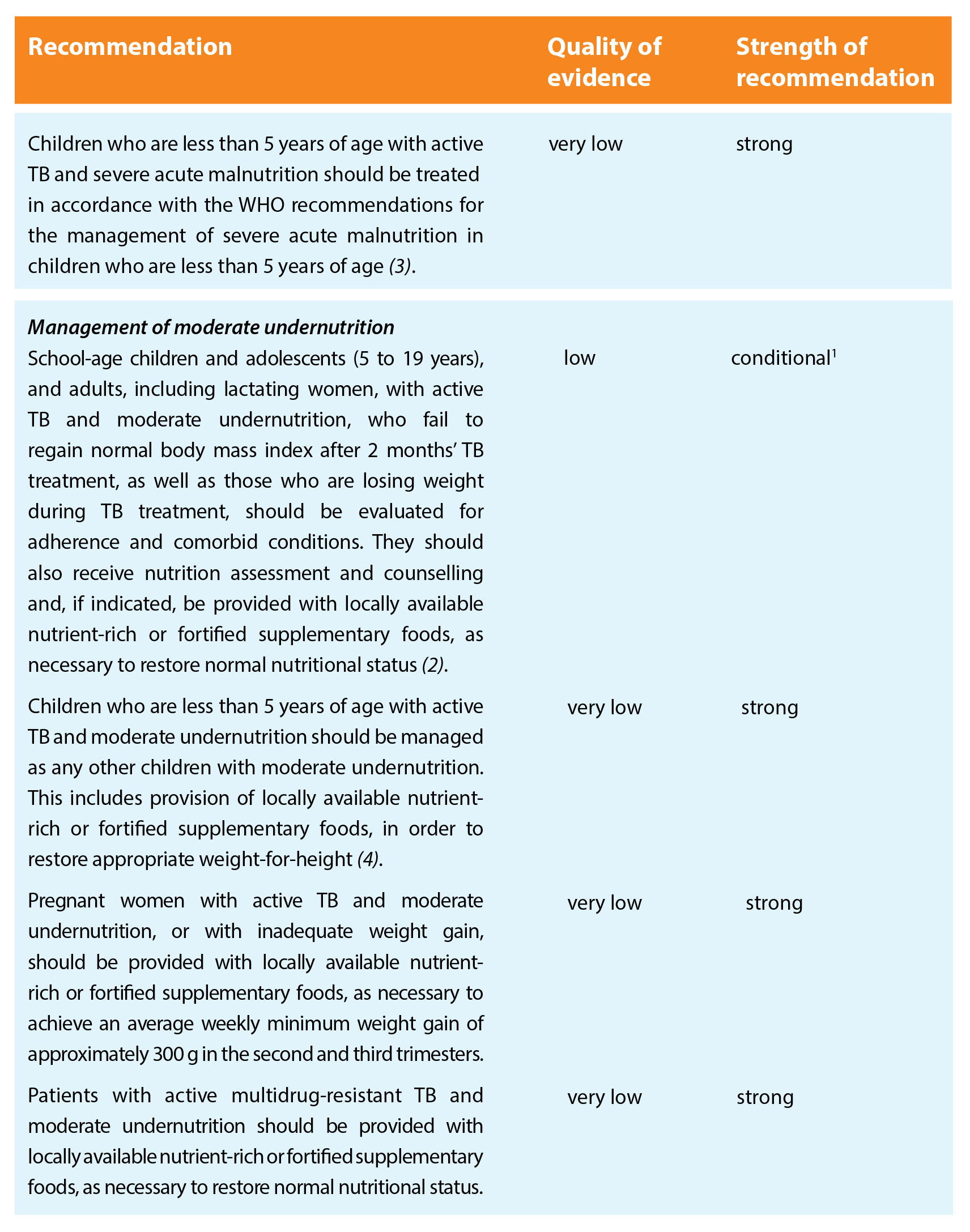
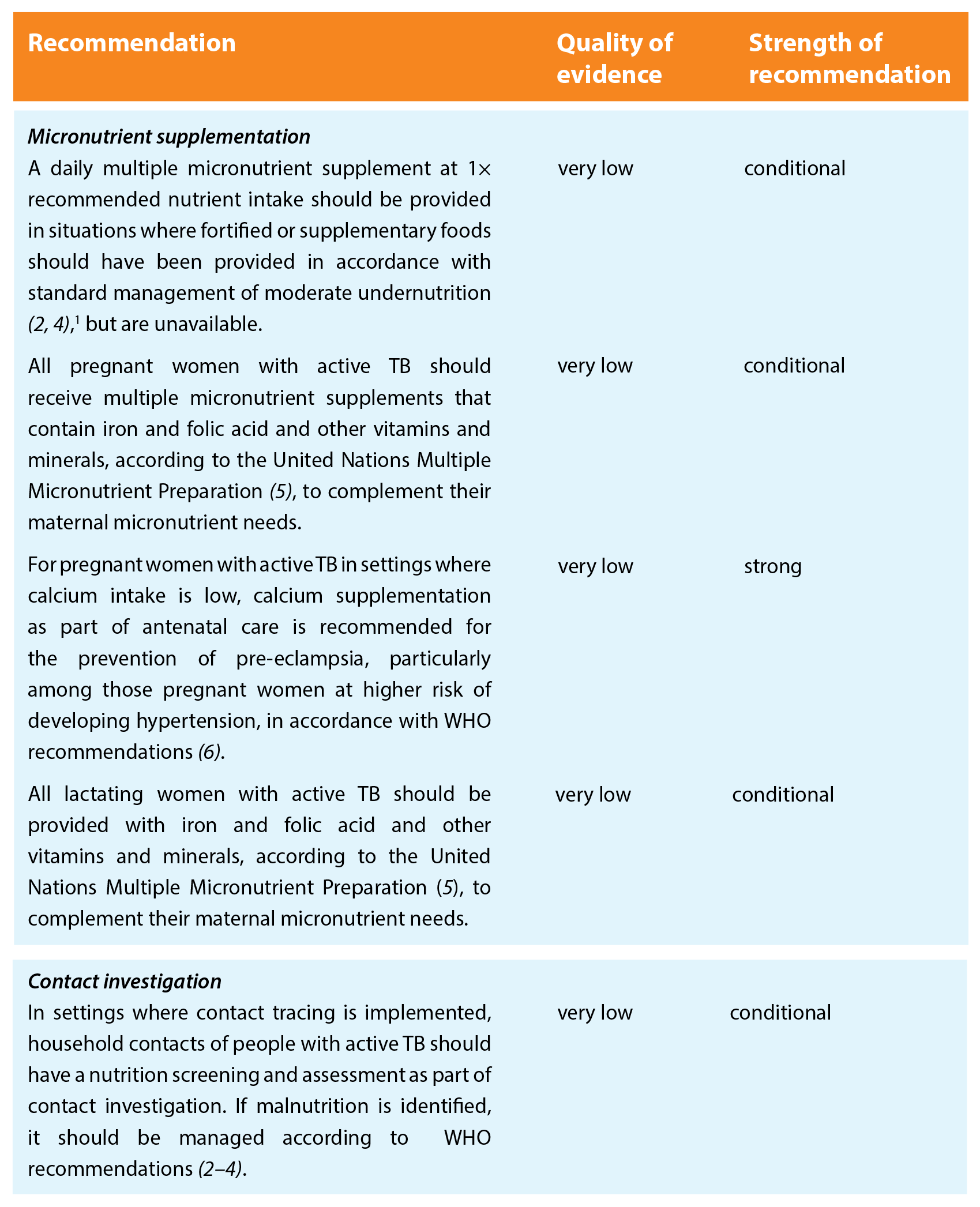
Key remarks
- There is no evidence that nutritional management of acute malnutrition of patients with active TB should be different than for those without active TB.
- Concerns about weight loss or failure to gain weight should trigger further clinical assessment (e.g. resistance to TB drugs, poor adherence, comorbid conditions) and nutrition assessment, in order to determine the most appropriate interventions.
- Closer nutritional monitoring and earlier initiation of nutrition support (before the first 2 months of TB treatment are completed) should be considered if the nutritional indicator is approaching the cut-off value for a diagnosis of severe undernutrition.
Research priorities
Guideline group members and stakeholders identified several research priorities to improve the body of evidence at the basic, clinical, epidemiological and operational levels, on the nutritional care and support for patients with TB.
¹ A WHO guideline is any document, whatever its title, containing WHO recommendations about health interventions, whether they be clinical, public health or policy interventions. A recommendation provides information about what policy-makers, health-care providers or patients should do. It implies a choice between different interventions that have an impact on health and that have ramifications for the use of resources. A full guideline is one that provides complete coverage of a health topic or disease. It is expected to include recommendations in relation to all aspects of the topic (e.g. surveillance, diagnosis, public health and clinical interventions) and to be fully based on systematic reviews of the evidence for each aspect. All publications containing WHO recommendations are approved by the WHO Guidelines Review Committee.
¹ A guiding principle in health is a rule that has to be followed, or that may be desirable to follow, and cannot be proved or contradicted, unless propositions are made that are even clearer. It is a comprehensive and fundamental law, doctrine or assumption guiding health care and is understood by its users as the essential characteristics of health care and its designed purpose. A respect of the principles is needed in order for the health care to be used effectively. A guiding principle reflects a set of values that contextualize the provision of care in programmatic settings. Such values cannot be subjected to formal research but reflect preferences regarding public health approaches and goals. The principles are intended to inform and assist national technical groups and international and regional partners providing health care.
¹ A strong recommendation is one for which the guideline development group is confident that the desirable effects of adherence outweigh the undesirable effects. Implications of a strong recommendation for patients are that most people in their situation would desire the recommended course of action and only a small proportion would not. Implications for clinicians are that most patients should receive the recommended course of action, and adherence to this recommendation is a reasonable measure of good-quality care. With regard to policy-makers, a strong recommendation means that it can be adapted as a policy in most situations, and for funding agencies it means the intervention represents an appropriate allocation of resources (i.e. large net benefits relative to alternative allocation of resources).
¹ A conditional recommendation is one for which the guideline development group concludes that the desirable effects of adherence probably outweigh the undesirable effects, although the trade-offs are uncertain. Implications of a conditional recommendation for patients are that, while many people in their situation would desire the recommended course of action, a considerable proportion would not. Implications for clinicians are that they should help patients make a decision that is consistent with their values. With regard to policy-makers, a conditional recommendation means that there is a need for substantial debate and involvement from stakeholders before considering the adoption of the recommendation, and for funding agencies it means that the intervention may not represent an appropriate allocation of resources (i.e. alternative uses of resources may produce greater benefits).
¹ Pyridoxine supplementation is recommended along with isoniazid treatment for all pregnant (or breastfeeding) women, as well as for people with conditions such as HIV infection, alcohol dependency, malnutrition, diabetes, chronic liver disease or renal failure. Pyridoxine provision together with isoniazid treatment was not analysed for this guideline.
 Retour
Retour