Liens transversaux de livre pour 2.2.4 Moderate complexity automated NAATs
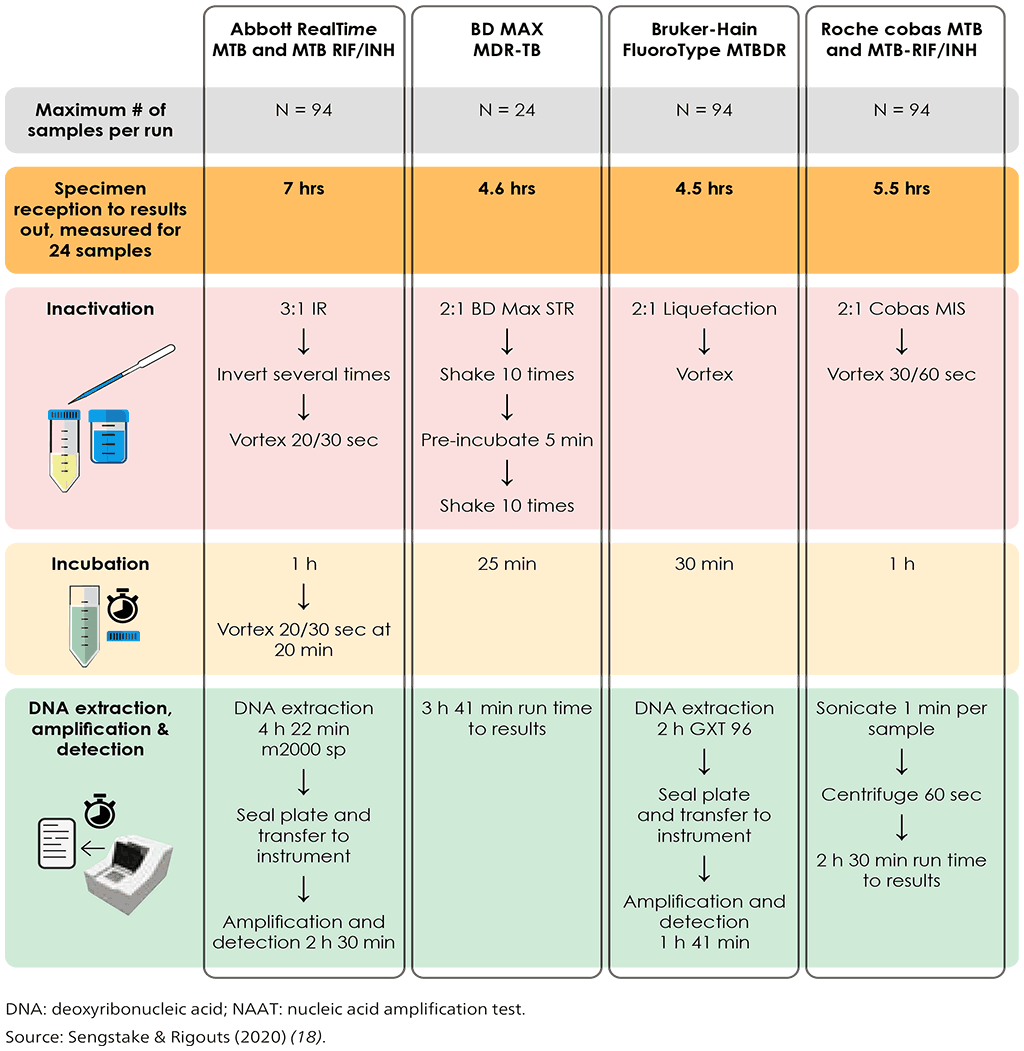
The moderate complexity automated NAATs class of tests includes rapid and accurate tests for the detection of pulmonary TB from respiratory samples. Overall pooled sensitivity for TB detection was 93.0% (95% confidence interval [CI]: 90.9–94.7%) and specificity 97.7% (95% CI: 95.6–98.8%) (Tables 3.1–3.4 in Section 3). Moderate complexity automated NAATs are also able to simultaneously detect resistance to both RIF and INH, and are less complex to perform than phenotypic DST and LPAs. After the sample preparation step, the tests are largely automated. Overall pooled sensitivity for detection of RIF resistance was 96.7% (95% CI: 93.1–98.4%) and specificity was 98.9% (95% CI: 97.5–99.5%). Fig. 2.1 illustrates the procedures for each test.
Overall pooled sensitivity for detection of INH resistance was 86.4% (95% CI: 82.8–89.3%) and specificity was 99.2% (95% CI: 98.1–99.7%). These assays offer high-throughput testing and are suitable for high workload settings, so could potentially be used in areas with a large population density or high TB prevalence. However, this class of tests is primarily for laboratory settings, and will require a reliable and rapid system for sample referral and result reporting. Moderate complexity automated NAATs may already be used programmatically for other diseases (e.g. severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], HIV, and hepatitis B and C) which could potentially facilitate implementation of TB testing on shared platforms. Information sheets summarizing the individual technologies in this class are available in Web Annex A. A detailed comparison of the different products is also available (17).
WHO has made the following recommendation (7):
- In people with signs and symptoms of pulmonary TB, moderate complexity automated NAATs may be used on respiratory samples for detection of pulmonary TB, RIF resistance and INH resistance, rather than culture and phenotypic DST.
Notes:
- This recommendation is based on evidence of diagnostic accuracy in respiratory samples of adults with signs and symptoms of pulmonary TB.
- The recommendation applies to PLHIV (studies included a varying proportion of such people). Performance on smear-negative samples was reviewed but was only available for TB detection, not for resistance to RIF and INH. Data stratified by HIV status were not available.
- The recommendation applies to adolescents and children based on the generalization of data from adults. An increased rate of indeterminate results may be found with paucibacillary TB disease in children.
- Extrapolation for use in people with extrapulmonary TB and testing on non-sputum samples was not considered because data on diagnostic accuracy of technologies in the class for non-sputum samples were limited.
Fig. 2.1. Summary of testing procedures for the newly endorsed moderate complexity automated NAATs
 Retour
Retour