Liens transversaux de livre pour 4.2 HIV treatment and care for people living with HIV diagnosed with TB
WHO recommends that all people with AHD should receive a package of interventions including screening, treatment and/or prophylaxis for major opportunistic infections, rapid ART initiation and intensified adherence support interventions, as listed in Annex 4. Several of these interventions are outlined in other parts of this operational handbook, such as the use of TB screening and diagnosis, including with LF-LAM, the provision of TPT, initiation of ART, and provision of co-trimoxazole prophylaxis. Yet, there are further elements of the AHD package of care that could be integrated within services for HIV-associated TB, including interventions to address cryptococcal disease, as well as histoplasmosis where the epidemiology indicates.
Antiretroviral therapy greatly improves the survival rate and the quality of life of people with HIV-associated TB, prevents HIV transmission and is central to HIV and TB treatment and prevention. ART is recommended for all people living with HIV regardless of WHO clinical stage and CD4 cell count. This section relates to specific considerations for ART initiation among people with diagnosed or presumptive TB. For details on ART regimens, see the latest WHO guidance on ART: WHO Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach (17).
4.2.1 Rapid ART initiation, including among people with HIV and TB symptoms
WHO guidelines recommend initiating ART within 7 days after HIV diagnosis and that people with advanced HIV disease are given priority for assessment and ART initiation. The guidelines further recommend offering ART initiation on the same day to people who are ready to start (17). However, immediate ART initiation is contraindicated among people living with HIV who may have central nervous system infections with risk of life-threatening IRIS, such as TB meningitis or cryptococcal meningitis (see Box 4.3).
People presenting with HIV for the first time or those returning to HIV care should have their history taken and undergo clinical examination to evaluate for TB and other opportunistic infections before rapid ART is offered. Tuberculosis symptoms are common among people with HIV. This should not be a reason to delay start of ART (17). Evidence from a systematic review which included four randomized controlled trials, found that 7–47% of people living with HIV presenting for same-day ART had TB symptoms (140-144). Very little information is available on the potential harm of sameday ART initiation in the presence of TB symptoms; however, the review supported the feasibility of this approach. Experience in implementing rapid ART initiation among people living with HIV with TB symptoms (except for TB meningitis) in countries such as Malawi, also suggests that this approach is feasible (145). Therefore, countries may consider initiating ART among people living with HIV who present signs and symptoms suggestive of TB, except for those with symptoms suggestive of meningitis,⁶ while rapidly investigating for TB, with close follow-up within 7 days to initiate TB treatment if TB is confirmed (17). The approach to rapid ART initiation must include an assessment for and related clinical management of advanced HIV disease.
4.2.2 ART initiation in people with HIV-associated TB
Since 2021, WHO has recommended that ART is initiated as soon as possible and within 2 weeks of starting TB treatment for all people with HIV-associated TB who are not on ART, regardless of CD4 cell count, with the exception of people with signs and symptoms of meningitis (17). Caution is needed with people living with HIV with TB meningitis, as detailed in Box 4.3, as evidence shows that earlier ART is associated with more severe adverse events when compared with initiation of antiretroviral therapy 2 months after the start of TB treatment among people with HIV-associated TB meningitis (146). ART should therefore be delayed for at least 4 weeks (and initiated within 8 weeks) after treatment for TB meningitis is initiated. Corticosteroids should be provided as an adjuvant treatment for TB meningitis. Furthermore, data are limited on the provision of ART to people with MDR-TB within 2 weeks, but initiation of ART in this population is recommended as soon as possible, and at most within 8 weeks. Fig. 4.3 summarizes the time frame for ART initiation among people with TB.
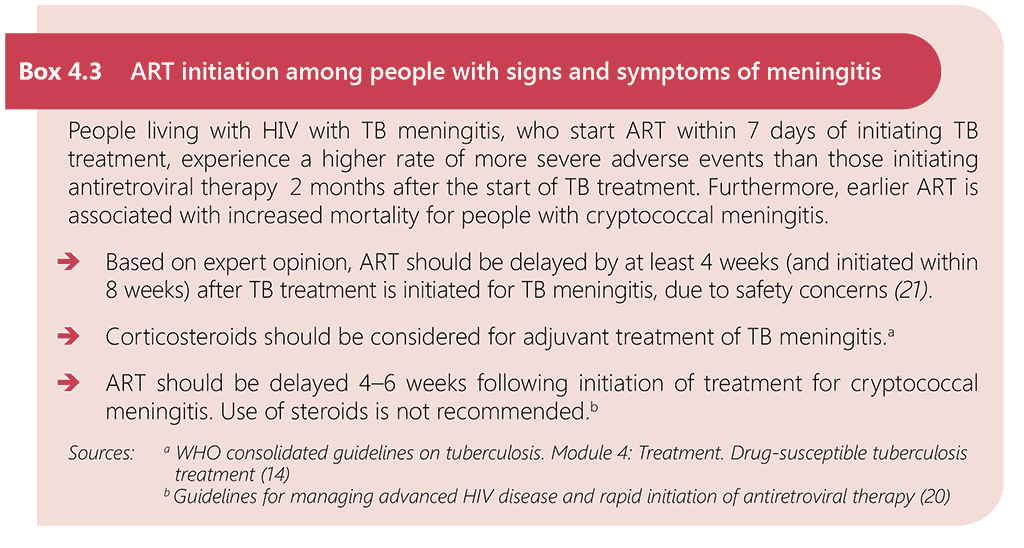
Fig. 4.3. Time frame for ART initiation
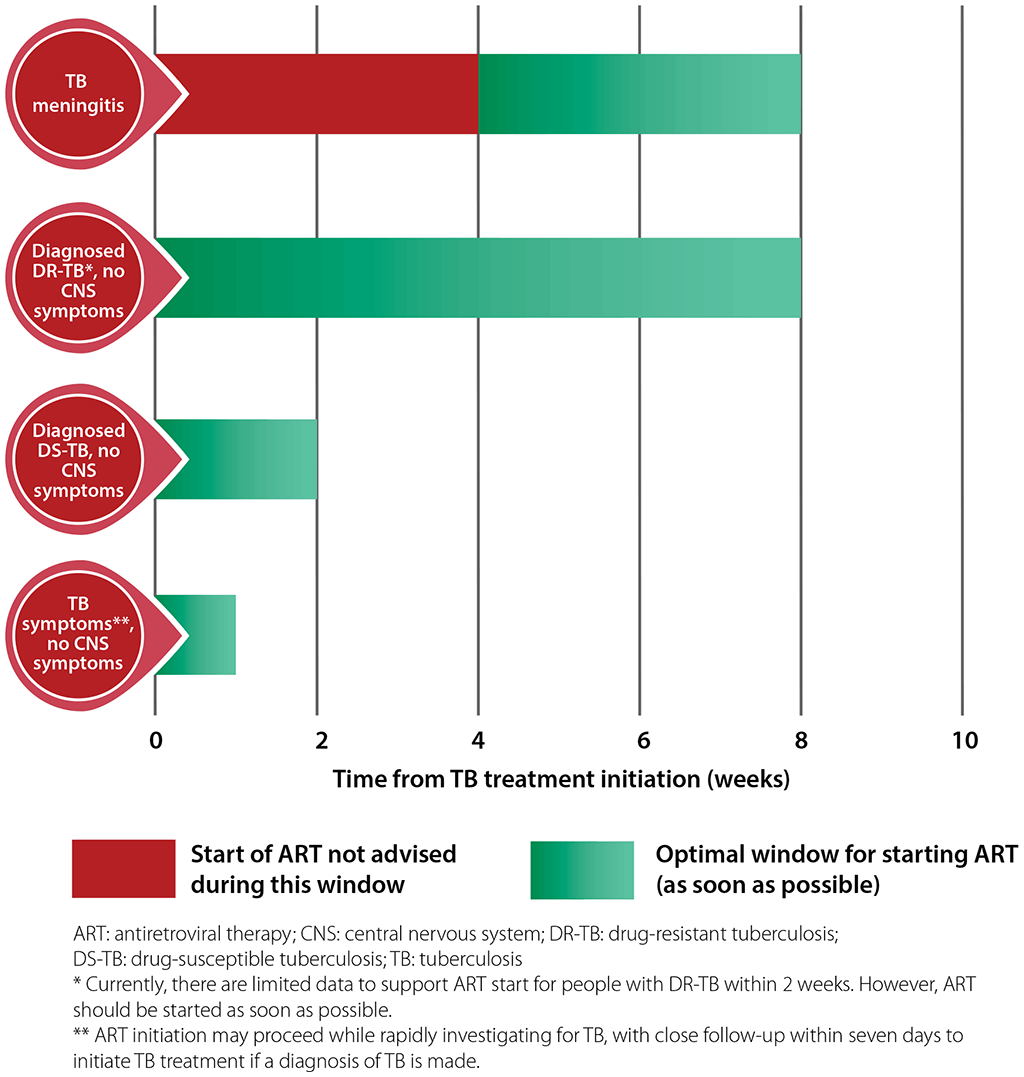
Tables 4.1 and 4.2 summarize current WHO recommendations for first-line and second-line ART regimens. For further details, see the latest WHO guidelines (currently the Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach) (17).
Table 4.1. First-line ART regimens for adults and adolescents (17)
a WHO Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach, 2021 update discusses toxicity considerations for pregnant and breastfeeding women.
b EFV-based ART should not be used in settings with national estimates of pretreatment resistance to NNRTIs of 10% or higher. In settings with high HIV drug resistance prevalence and where DTG is unavailable or unsuitable due to toxicity, a boosted PI-based regimen (PI/r) should be used. The choice of PI/r will depend on clinical and programmatic characteristics. Among PI/r options, only LPV/r with adjusted dosage is suitable for people using TB regimens containing rifampicin. Alternatively, HIV drug resistance testing should be considered, where feasible, to guide first-line regimen selection.
Table 4.2. Second-line ART regimens for adults and adolescents (17)
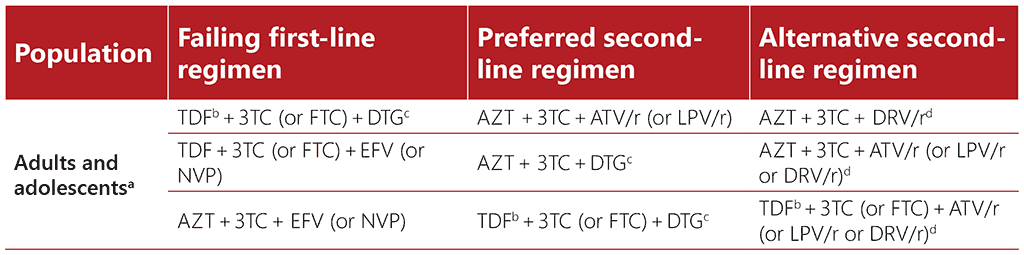
3TC: lamivudine; ATV/r: atazanavir/ritonavir; AZT: zidovudine; DRV/r: darunavir/ritonavir; DTG: dolutegravir; EFV: efavirenz; FTC: emtricitabine; LPV/r: lopinavir/ritonavir; NVP: nevirapine; TDF: tenofovir disoproxil fumarate
a Sequencing if a PI is used in first-line ART: TDF + 3TC (or FTC) + ATV/r (or LPV/r, or DRV/r, depending on programmatic considerations) in first-line ART should be sequenced to AZT + 3TC + DTG in second-line ART.
b See WHO Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach, 2021 update for HIV drug resistance considerations when using TDF+3TC+DTG in second-line ART following failure of TDF+3TC (or FTC)+EFV.
c TAF can be used as an alternative NRTI for children and in special situations for adults (see section on TAF in first-line ART, in WHO Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach, 2021 update).
d RAL+LPV/r can be used as an alternative second-line regimen for adults and adolescents.
People with HIV-associated TB should be closely followed up to monitor for adverse events related to co-treatment. IRIS and other adverse events require prompt assessment and management, especially among children and pregnant or breastfeeding women. IRIS is more common in people with TB who have started on ART, with lower CD4 cell counts, but with the exception of central nervous system IRIS (see Box 4.3), it is usually mild, self-limited and should normally not be a reason to interrupt ART (147, 148).
Drug–drug interactions may occur in people with HIV-associated TB and people with HIV who receive TPT, in particular when rifampicin-based regimens are used. As an example, rifampicin is known to lower plasma concentrations of the first-line antiretroviral drug dolutegravir. WHO therefore recommends adjusting the dose by offering 50 mg of dolutegravir twice per day (instead of a single daily dose of 50 mg). Key considerations for drug–drug interactions are described in Section 3.4 (TB treatment), whilst Section 3.5 (TB prevention) also gives an overview of drug–drug interactions between HIV and medicines used for TB treatment or prevention. To check for drug–drug interactions from a comprehensive list of antiretrovirals and co-medications the reader is directed to the University of Liverpool’s HIV Drug Interactions Group resource (www.hiv-druginteractions.org/) and the associated Android and iOS apps (search “HIV iChart”).
HIV programmes and TB programmes should ensure that people with TB who receive a new diagnosis of HIV are offered ART as early as possible, preferably within integrated services or within TB health facilities in settings with a high burden of HIV and TB. Referral to HIV services remains an alternative but relies on sound referral systems and the patient’s ability to afford other costs such as transport and the loss of wages. HIV programmes and TB programmes should work together to guarantee ART to all people with HIV-associated TB in as decentralized a manner as possible (7).
A comprehensive package of prevention, diagnosis, treatment and care interventions should be provided to all people living with HIV. A continuum of care should also be provided to people living with HIV who are receiving or who have completed their antituberculosis treatment through integrated services or strengthened referral systems. Evidence has shown that linking TB and HIV prevention, diagnosis, treatment and care services may generate synergies, strengthen both programmes and scale up the delivery of these interventions to people with HIV-associated TB (41).
Programmes should establish mechanisms for adequate monitoring, including pharmacovigilance and surveillance, for drug–drug interactions. For further guidance on this, please refer to the Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach (17). Key considerations include adequate training of healthcare personnel and programme managers to deliver integrated TB and HIV services (crosstraining), and HIV and maternal, newborn and child health services, including for children, adolescents and pregnant women; co-location of services and establishing an integrated supply chain, laboratory and information systems. Coordination between TB and HIV programmes to deliver these services is critical. Also needed are community engagement, patient education, engagement of adherence counsellors and social workers, and peer support for early recognition of IRIS and adverse events, and to support the retention of and adherence to co-treatment.
4.2.3 Co-trimoxazole prophylaxis
Co-trimoxazole is a fixed-dose combination of two antimicrobial agents (sulfamethoxazole and trimethoprim) used to treat or prevent a variety of bacterial, fungal and protozoan infections, including Pneumocystis jirovecii pneumonia, toxoplasmosis and malaria. Co-trimoxazole prophylaxis is a feasible, well-tolerated and inexpensive intervention to reduce HIV-related morbidity and mortality among people living with HIV and can be administered concomitantly with ART and TB treatments. Although co-trimoxazole has low rates of toxicity, skin rash (including Stevens-Johnson syndrome), reactions of the blood and blood-forming organs, and liver toxicity, have been reported (149). WHO guidelines recommend providing lifelong co-trimoxazole prophylaxis to everyone living with HIV, regardless of CD4 cell count, in settings where severe bacterial infections or malaria are highly prevalent. This is an important consideration for linkage to ongoing treatment and care following completion of TB treatment.
Co-trimoxazole prophylaxis should be provided for people with HIV-associated TB, regardless of their CD4 cell count, as an integral component of the HIV care package. HIV programmes and TB programmes should establish a system to provide co-trimoxazole prophylaxis to all people living with HIV who have TB disease. Co-trimoxazole is an off-patent drug and is widely available in resourcelimited settings (17).
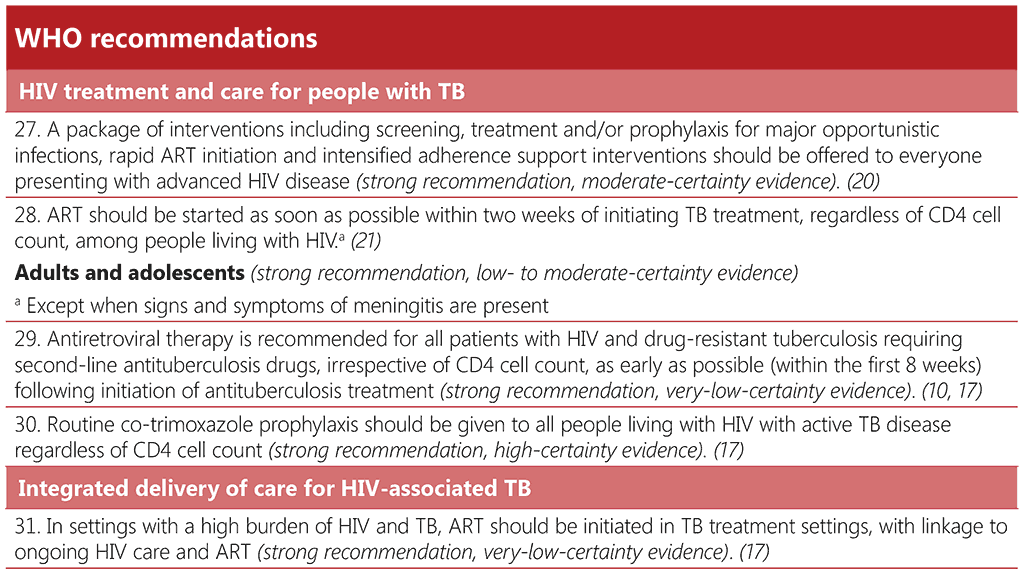
Given the importance of co-trimoxazole, TB and HIV programmes should work together to overcome any barriers to its provision, which may include supply chain and management issues leading to stockouts; imposing user charges for medication and/or monitoring; inadequate training, supervision and/or mentoring of healthcare workers; low coverage of HIV testing services; and lack of coordination across programmes.
4.2.4 Other opportunistic infections: cryptococcal disease and histoplasmosis
4.2.4.1 Cryptococcal disease
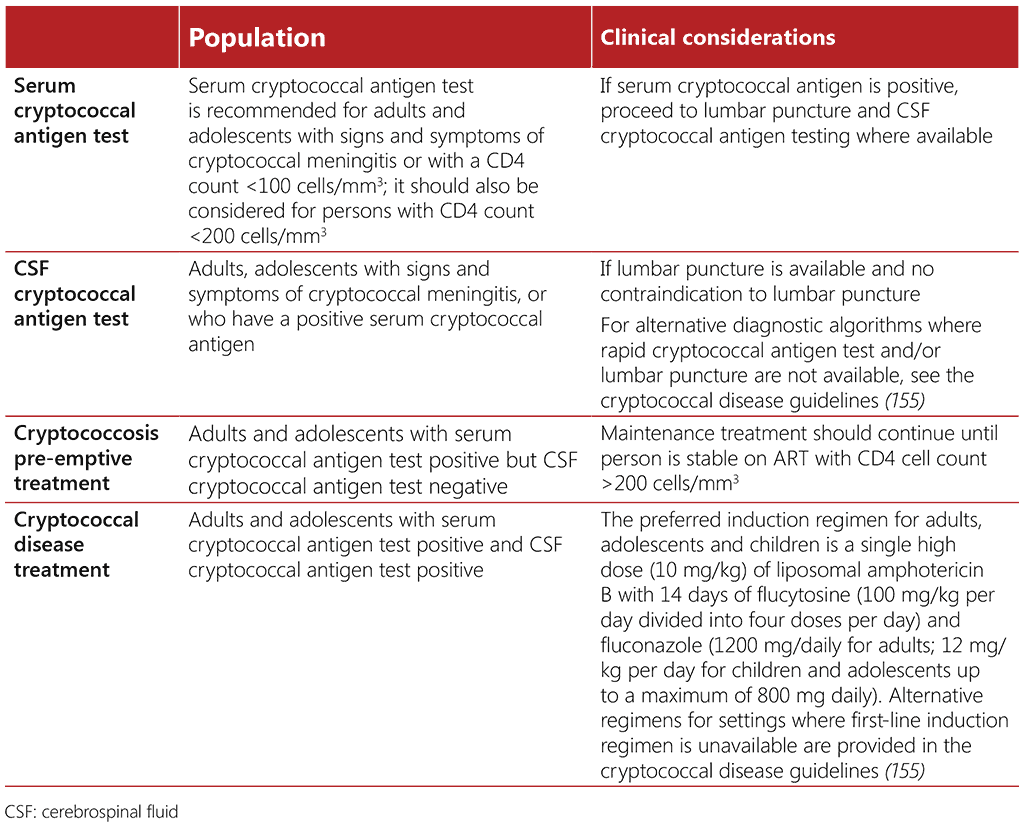
Cryptococcal disease causes severe morbidity among people living with advanced HIV disease and is a major contributor to disability and mortality (20, 150-153). Up to 90% of HIV-related cryptococcal disease is cryptococcal meningitis (151). A systematic review found a TB prevalence of 19% among people living with HIV testing positive for the cryptococcal antigen (154). Early diagnosis and preemptive treatment of cryptococcal disease is key to reducing mortality among people living with HIV. Core aspects of testing and treatment for cryptococcal disease are summarized in Table 4.3.
Table 4.3. Testing and treatment for cryptococcal disease
Cryptococcal antigen screening
Adults and adolescents with advanced HIV disease should be screened for cryptococcal disease using serum, plasma or whole blood cryptococcal antigen testing. Everyone testing positive for cryptococcal antigen during screening should be carefully evaluated for signs and symptoms of meningitis; those presenting with signs or symptoms should have a lumbar puncture. Where feasible, those who test positive for serum cryptococcal antigen but who do not have signs or symptoms of meningitis should also have lumbar puncture, with CSF examination and cryptococcal antigen assay (or India ink if cryptococcal antigen assay is not available) to exclude cryptococcal meningitis (33). When performing a lumbar puncture to assess for cryptococcal meningitis in people with presumed or diagnosed TB, samples may also be taken to assess other causes of meningitis, including TB meningitis.
Rapid cryptococcal antigen assay in CSF, serum, plasma or whole blood (depending on access to lumbar puncture) is preferred, based on the much higher diagnostic accuracy of these rapid cryptococcal antigen assays versus the India ink test and the fact that these rapid assays depend less on the healthcare provider’s skills. Advantages of the lateral-flow assay over the latex agglutination assay include its rapid (<10 min) turnaround time, cost-effectiveness, minimal training requirements and laboratory infrastructure, no need for refrigerated storage and higher clinical and analytical sensitivity (33).
Detailed guidance on screening and diagnostic algorithms for cryptococcal disease, depending on the availability of rapid cryptococcal antigen test and lumbar puncture, is outlined in the WHO Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV (34).
Pre-emptive treatment of cryptococcal disease
All individuals who screen positive for serum cryptococcal antigen, but have a negative CSF cryptococcal antigen test, should receive pre-emptive antifungal therapy, consisting of fluconazole 800–1200 mg/day for adults and 12 mg/kg per day for adolescents for 2 weeks, followed by consolidation and maintenance fluconazole therapy, as per the treatment regimen (33). When cryptococcal antigen screening is not available, fluconazole primary prophylaxis should be given to adults and adolescents living with HIV who have a CD4 count <100 cells/mm3. This may also be considered at a higher CD4 cell count threshold of <200 cells/mm3 (33).
Treatment of cryptococcal meningitis
Treatment of cryptococcal meningitis consists of a 2-week induction phase, followed by an 8-week consolidation phase, and a maintenance phase to continue until immune reconstitution (CD4 >200 cells/mm3) and suppression of viral load on ART. Steroids should not routinely be used for people with cryptococcal meningitis due to an increase in adverse events and delayed clearance of fungus from CSF (156). Further guidance on treatment regimens is provided in the WHO Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV (155).
Service delivery
Given its high morbidity among people with HIV, and the high rate of TB among people screening positive for cryptococcal antigen, it is important to address cryptococcal disease in people presenting with advanced HIV disease. Depending on the infrastructure and resources available, TB services offer an opportunity to expand access to screening, diagnosis, treatment and prevention of cryptococcal disease in both inpatient and outpatient settings.
The relative advantage of various approaches to screening for cryptococcal disease should be considered according to context. In resource-limited settings with limited laboratory infrastructure, task sharing has been found to overcome human resource limitations. A study in Lesotho found that cryptococcal antigen screening by lay counsellors followed by pre-emptive fluconazole treatment for asymptomatic people with a positive cryptococcal antigen screening test, or referral to hospital for those with symptomatic cryptococcal disease, was feasible (157). This suggests that integration of screening and pre-emptive treatment for cryptococcal disease within TB services may also be feasible, and may be the preferred approach, especially when point-of-care CD4 cell count is available, enabling same-day initiation of fluconazole among those who screen cryptococcal antigen-positive.
4.2.4.2 Histoplasmosis
Histoplasmosis has a high endemicity in certain areas of the Americas, where it is most frequently encountered (16). It is also diagnosed in certain countries of Asia (China, India, Indonesia, Japan, Malaysia, Singapore, Thailand, and Viet Nam) and Africa (Central African Republic, Congo, Côte d’Ivoire, Democratic Republic of the Congo, Gambia, Guinea-Bissau, Liberia, Senegal, South Africa, and Uganda) (16). Among people living with HIV, the most frequent clinical presentation of this disease is disseminated histoplasmosis. Symptoms of disseminated histoplasmosis are nonspecific and may be indistinguishable from those of other infectious diseases, especially TB, thus complicating diagnosis and treatment (158).
Lack of access to appropriate antifungal therapies, in vitro diagnostics for rapid detection of histoplasmosis and co-occurrence with TB, may affect clinical outcomes and contribute to the high mortality of people living with HIV (159, 160). If histoplasmosis is clinically suspected, WHO recommends diagnostic testing using antigen detection assays (16).
It is important to review potential ART options for people with TB and histoplasmosis and to make necessary adjustments as recommended in the Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach (17). Because of the potential decrease in itraconazole levels related to some types of antiretroviral therapy, itraconazole drug levels should be monitored if possible (161). When histoplasmosis is not controlled because of interactions with rifampicin and itraconazole, clinicians may consider extending the duration of amphotericin B induction therapy, depending on the local context; other options include once-weekly courses of amphotericin B, increasing the itraconazole dose and monitoring the blood level and toxicity, as well as considering using other azole drugs (posaconazole, voriconazole, or fluconazole).
Treatment may need to be revised for people experiencing toxicity, drug–drug interactions, or for those with resistance profiles requiring protease inhibitors or second-line anti-TB drugs. When possible, antiretroviral resistance genotyping may assist clinical decisions. Itraconazole serum level testing may not be available in some areas.
5 For adults and adolescent, advanced HIV disease is defined as CD4 cell count <200 cells/mm3 or WHO stage 3 or 4 event. See:
Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy, July 2017 (https://apps.who.int/iris/handle/10665/255884, accessed 31 August).
⁶ Common signs and symptoms of meningitis include stiff neck, high fever, sensitivity to light, confusion, headaches and vomiting (https://www.who.int/news-room/fact-sheets/detail/meningitis).
 Retour
Retour