Liens transversaux de livre pour 4.2.9 Area 9 – Recording and reporting

Step 9.1 – Review and revise request for examination and reporting forms
In an ideal system, forms to request TB infection testing, record TB infection results, and request further medical evaluation and CXR are all part of an integrated electronic system – streamlining information flow and minimizing providers’ time (and errors) in providing information. It is suggested that forms should be short and simple, collecting only the most essential and actionable information.
Reporting results of TB infection testing, medical evaluation, CXR and other testing should also be streamlined, and ideally should be digitized to facilitate rapid referral and analysis. The Prevent TB mobile application and digital platform (Fig. 4.3) is expected to facilitate systematic recording and reporting as well as monitoring of data across the cascade of care for TB infection. Where paper forms are used, it is suggested that patients always be given a copy of the results.
Fig. 4.3. The Prevent TB digital platform
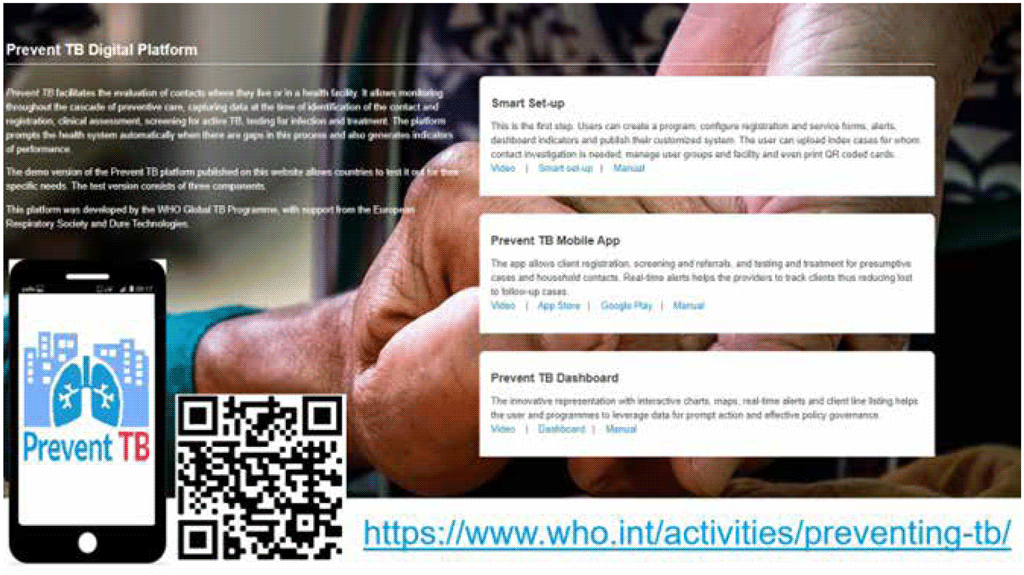
Source: WHO (67).
IGRA results should be reported qualitatively (positive or negative) and quantitatively (TB antigen – Nil = N IU/mL) to facilitate interpretation. Importantly, the method used should be reported.
Step 9.2 – Review and revise laboratory and clinical registers
Registries in laboratories and medical records should be standardized. In countries that have adopted electronic medical records, a field for the new tests is required. WHO is currently revising its reporting framework (68).
If surveillance systems incorporate new tests, then forms need to include fields for those new tests.
 Retour
Retour