Liens transversaux de livre pour 3.4 TB treatment
3.4.1 WHO-recommended treatment regimens for adults with drug-susceptible TB
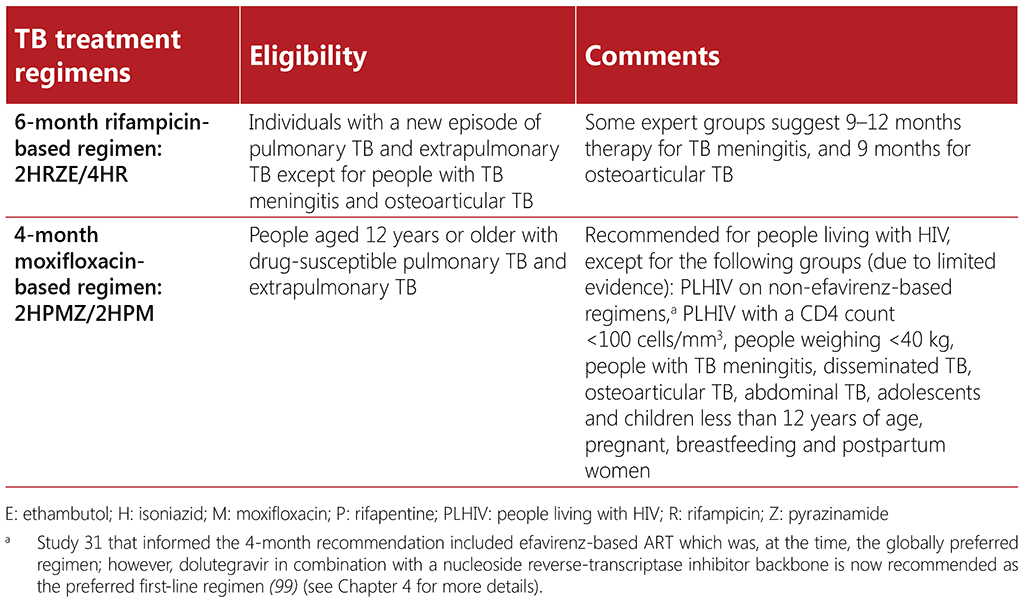
WHO currently recommends two treatment regimens for adults with drug-susceptible TB (DS-TB): a 6-month regimen composed of 2 months of isoniazid (H), rifampicin (R), pyrazinamide (Z) and ethambutol (E), followed by 4 months of isoniazid and rifampicin (2HRZE/4HR) and a 4-month regimen composed of 8 weeks of isoniazid, rifapentine (P), moxifloxacin (M) and pyrazinamide, followed by 9 weeks of daily isoniazid, rifapentine, and moxifloxacin (2HPMZ/2HPM). Both the 6-month and 4-month regimens are applicable to people living with HIV except for certain subpopulations as indicated. Aligned to the recommendation on the 6-month regimen, WHO has published four additional recommendations on dosing frequency, the use of fixed dose combination tablets and extension of the intensive phase. Recommendations on treatment regimens for DS-TB are outlined in full in the WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-susceptible tuberculosis treatment (14) as well as the WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents (8). Two additional recommendations on the use of corticosteroids for the treatment of TB meningitis or TB pericarditis are also included in the WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-susceptible tuberculosis treatment (14). Treatment regimens for drug-susceptible TB are summarized in Table 3.3.
3.4.1.1 Six-month TB treatment regimen for adults living with HIV
The 6-month regimen can be used in all subgroups, including people living with HIV. This regimen can also be used for people with extrapulmonary TB, except those with TB affecting the central nervous system or with osteoarticular forms of TB, for whom expert groups suggest a longer duration of therapy. Despite its familiarity, safety and efficacy, many people find the 6-month regimen difficult to complete due to its length. The 6-month regimen is available in fixed-dose combination tablets which helps decrease the pill burden; an important consideration for people living with HIV and other comorbidities (93).
The interactions of rifampicin (the mainstay of TB treatment) with ART are of concern in HIVassociated TB. When the 6-month regimen is used, these drug interactions may result in decreased concentrations of antiretroviral drugs. Rifampicin is known to lower plasma concentrations of the first-line antiretroviral drug dolutegravir. This has led to concerns about efficacy of dolutegravir when co-administered with rifampicin, and the subsequent development of HIV drug resistance due to lower levels of dolutegravir. In such cases, WHO guidelines recommend adjusting the dose by offering 50 mg of dolutegravir twice per day (instead of a single daily dose of 50 mg) (17). These recommendations are still in place, although evidence that doubling the dose of dolutegravir might not be necessary is emerging. A study from Botswana demonstrated the efficacy and safety of a standard dose dolutegravir-based regimen compatible with an efavirenz-based regimen for people living with HIV-associated TB who received rifampicin (94).
Standard, rifampicin-containing anti-TB treatment is recommended in combination with efavirenzbased ART, without the need for dose adjustment. Conversely, rifampicin is contraindicated in combination with nevirapine and protease inhibitors. Rifabutin is a less potent inducer of the cytochrome P450 system which may be considered in people on some ART regimens that include nevirapine or a protease inhibitor, with close monitoring for safety and tolerability (7, 95). Rifabutin is also the preferred rifamycin to be administered alongside OAMT (96).
3.4.1.2 Four-month TB treatment regimen for adults living with HIV
Study 31 provided the evidence that informed the WHO recommendation on the 4-month moxifloxacinbased regimen (10). This study included some people living with HIV (8%), most of whom were on ART (efavirenz-based regimens). However, people with a CD4 count of less than 100 cells/mm3 were excluded from the trial (97). Thus, sufficient evidence is available to support the use of the 4-month regimen when the CD4 count is above 100 cells/mm3 . Additional studies are needed to inform the use of the 4-month regimen in people living with HIV who are taking non-efavirenz-based ART regimens, and for people who have a CD4 count less than 100 cells/mm3 (98). In settings with a high background prevalence of fluoroquinolone resistance, fluoroquinolone drug susceptibility testing is recommended, which might pose a further barrier to scale-up in certain settings.
There may be challenges to implementation of the 4-month regimen initially, until rifapentine becomes more widely and readily available at costs comparable with rifampicin, and a fixed-dose combination (FDC) tablet is developed. At present, the overall pill burden will be higher for people who receive this 4-month regimen1 because no FDC tablet currently exists for the regimen and the dose of rifapentine is high (1200 mg). This may affect acceptability; however, this situation may change in future as uptake of this regimen improves, creating a demand for the regimen and its component medicines. Wider availability of the rifapentine formulation of 300 mg2 may decrease the pill burden and facilitate the implementation of this new regimen until the FDC tablet becomes available.
Table 3.3. Treatment regimens for drug-susceptible TB
3.4.2 WHO-recommended treatment regimens for drug-resistant TB
People with both HIV and multidrug-resistant TB face complicated clinical management, fewer treatment options and poorer treatment outcomes (100). Systematic reviews have shown an association between HIV and MDR-TB (101, 102). Outbreaks of multidrug-resistant TB among people living with HIV have been documented in hospital and other settings, especially in eastern Europe and central Asia and in southern African countries with a high HIV prevalence (103).
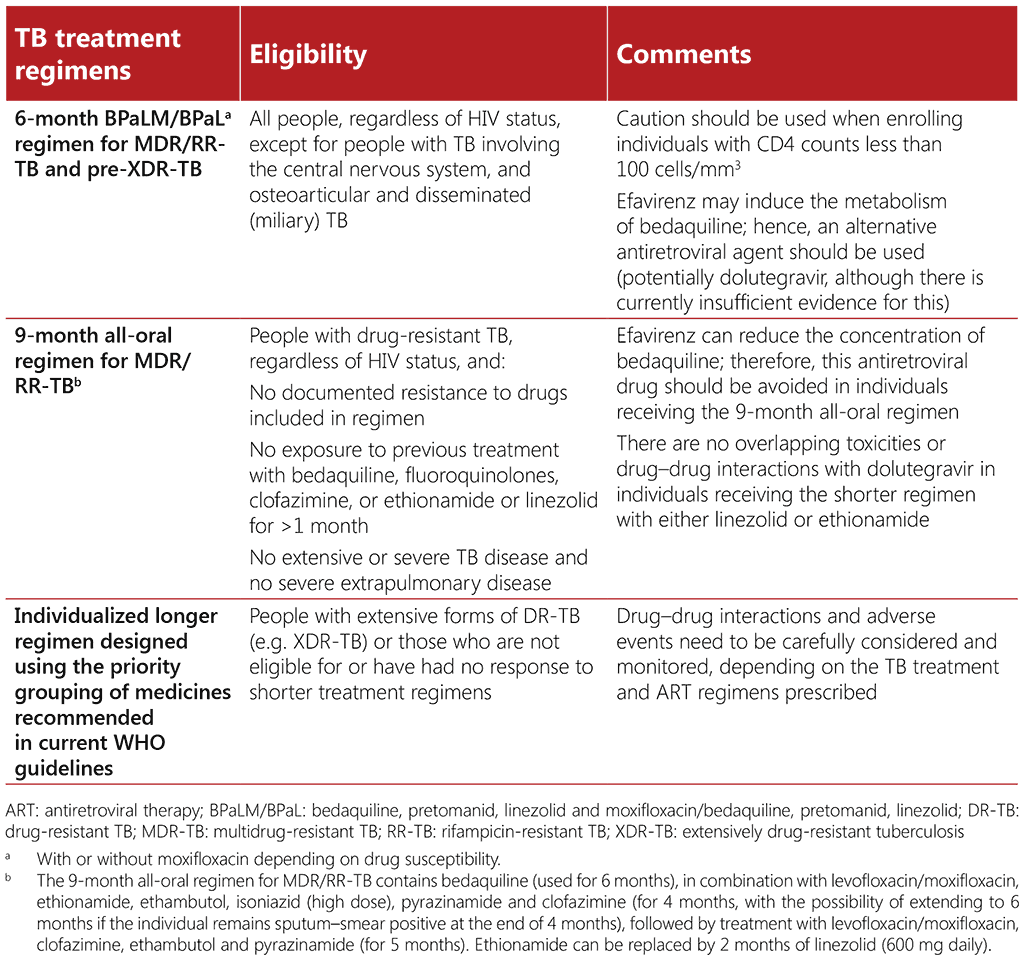
Table 3.4 below provides a brief overview of treatment regimens for drug-resistant TB; detailed guidance is published in the WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment, 2022 update (10) and the accompanying operational handbook (104). People living with HIV and drug-resistant TB are generally eligible for all the currently available TB treatment regimens, but due care should be taken to monitor for adverse events and drug– drug interactions. Detailed guidance on caution when administering treatment for drug-resistant TB together with ART is available in the WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment, 2022 update (104), and in the University of Liverpool’s HIV Drug Interactions resource³ (www.hiv-druginteractions.org).
Table 3.4. Treatment regimens for drug-resistant TB
3.4.3 TB treatment for people with HIV-associated TB and histoplasmosis
Histoplasmosis is highly endemic in some parts of the WHO Region of the Americas and is also reported in certain countries of Asia and Africa (16). People with HIV-associated TB who also have histoplasmosis should receive prompt treatment after diagnosis according to WHO treatment guidelines. Joint management of TB, HIV and histoplasmosis can be complex, with drug–drug interactions that may affect treatment. Rifampicin, in particular, results in reduced itraconazole levels, potentially leading to ineffective treatment for histoplasmosis (105). Clinicians may consider replacing rifampicin with rifabutin. More information on histoplasmosis can be found in Chapter 4, and in the Pan American Health Organization and WHO Guidelines for diagnosing and managing disseminated histoplasmosis among people living with HIV (16).
3.4.4 TB care and support
TB and HIV programmes should aim to deliver integrated TB and HIV care, preferably at the same time and location, with due consideration for the prevention of TB transmission. Early ambulatory care is recommended, including for the management of drug-resistant TB (DR-TB) using the latest recommended DR-TB regimens, because it complements the person-centred approach to the management of TB (10). Therefore, countries are encouraged to move towards a decentralized and ambulatory model of care. However, some people with HIV-associated TB may need to stay in hospital to receive treatment for TB. This would be the case, for instance, if the individual is seriously ill with HIV, develops severe immune reconstitution inflammatory syndrome (IRIS), has a severe form of DS-TB or DR-TB disease (e.g. meningitis, vertebral bone infection, pericarditis, miliary TB or severe TB lung disease with signs of respiratory distress/failure or sepsis), has other serious comorbidities (such as severe malnutrition or uncontrolled diabetes mellitus), is either very young or elderly or has serious adverse reactions to medication (106). In such situations, these individuals may need to be hospitalized until their condition stabilizes. Long hospitalization should not be routinely required for people on DR-TB treatment unless it is absolutely necessary from a medical standpoint. The treatment regimen should rarely require a person with DR-TB to be hospitalized because every attempt should be made to put the person on an all-oral regimen that they can receive as an outpatient. Furthermore, the individual should be kept in isolation while hospitalized only when no other options remain.
Social determinants of health are not only driving the HIV and TB epidemics but are also a challenge for affected people to adhere to treatment for any form of TB – particularly for people living with HIV. Persons with TB and HIV often experience stigma and discrimination in many areas of life, including work, social activities and family life. Social, economic, cultural and legal issues may pose additional barriers in accessing health care and being able to consistently follow medical advice. Consequently, it is important that the healthcare services are aware of all the barriers faced by people affected by TB and provide appropriate and comprehensive social support; furthermore, social protection measures to enable adherence and reduce economic hardship should be provided. Treatment adherence interventions that may be offered for people on TB treatment include material support (e.g. food, financial enablers, transport fees), psychological support, tracers such as home visits or digital health communication (e.g. SMS, telephone call) and medicine monitoring (14, 15). Interventions should be selected based on the assessment of the individual’s barriers to access, needs and preferences as well as available resources. Education and counselling on TB and its treatment should also be provided to all people with TB.
¹ Based on estimates by the Global Drug Facility for an average weight of 55–70 kg: 1358 tablets versus 728 for whole course of treatment.
² Rifapentine 150 mg and 300 mg are both included in the WHO model list of essential medicines: 23rd list (2023) (https://apps.who.int/iris/handle/10665/371090
³ The Liverpool Drug Interactions resources receive support from the pharmaceutical industry, the British HIV Association, the European AIDS Clinical Society, and the HIV Glasgow Congress. Editorial content is independent of financial support and is overseen by an independent international editorial board. For details, please see www.hiv-druginteractions.org. The evaluation methodology is published in Seden et al. PLoS One. 2017 Mar 23;12(3):e0173509.
 Retour
Retour