Liens transversaux de livre pour 9.6 Preparation for the introduction of new treatment regimens
Given the increased use of newer and repurposed medicines in combination MDR-TB regimens, aDSM is particularly important. aDSM defines the active and systematic clinical and laboratory assessment of patients on MDR-TB treatment to detect, manage and report suspected or confirmed drug toxicities (18). It applies the principles of active pharmacovigilance to the specific needs and context of NTPs, and is embedded within the routine patient monitoring function (e.g. treatment outcome cohort monitoring) of NTPs. The management of patient safety is an inherent part of aDSM, inseparable from its monitoring component. The recording and reporting activities of aDSM primarily target serious adverse events as a priority requirement, but any adverse event during treatment administration (whether or not it is related to drug toxicity) needs to be managed to limit harms to patients. MDR-TB treatment sites may also monitor nonserious adverse events that are of clinical significance or of special interest to the programme, as part of more comprehensive aDSM. In aDSM, in addition to the spontaneously reported reactions, adverse events are elicited as part of a patient monitoring plan that comprises a set of questions and often an array of laboratory or clinical tests at defined periods of time, before, during and after treatment (Web Annex 3).
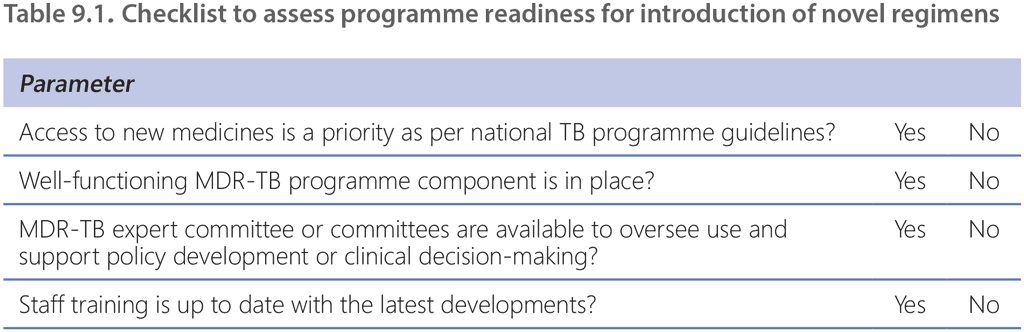
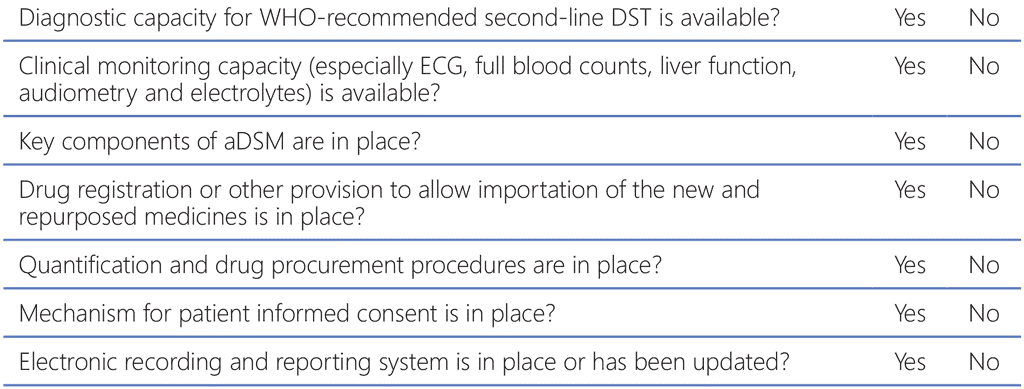
When planning important changes for the national TB treatment policy to align with the latest WHO recommendations, the programme needs to balance the will to provide the best possible options for patients according to the latest evidence against the programmatic circumstances and the implications of such changes (e.g. the need to re-train staff or obtain funds for reprogramming). Table 9.1 presents a checklist for considerations by the programme manager when implementing the MDR-TB regimens that are currently recommended. The programme must balance the need to provide access to new medicines for which the evidence is still incomplete with the need to protect patients from avoidable toxicity, the emergence of resistance to the new agents and observance of proper ethical conduct and respect for patient rights.
 Retour
Retour