Liens transversaux de livre pour A2.3 T-SPOT.TB – step by step
The T-SPOT.TB has multiple steps (4). These steps are detailed below and shown in Fig. A2.3.1 (for sample preparation and testing procedures) and in Fig. A2.3.2 (for interpretation).
Step 1. Blood-sampling procedures
The procedures for sample collection and preparation for T-SPOT.TB are different from those for QFT-Plus and WANTAI TB-IGRA. Whole blood samples should be collected into blood collection tubes (e.g. Vacutainer® CPT™ or lithium heparin tubes) and the tubes shaken to allow mixing of the heparin with the blood. The collected blood should be stored at room temperature (18–25 °C), and not refrigerated or frozen; about 8 mL of blood is needed. Next, working buffer and antibodies are added, which will bind T-cells (15-minute incubation at room temperature). Then, a bead reagent is added, which mediates the formation of antibody-mediated bead immune complexes (a further 15-minute incubation at room temperature).
Step 2. Peripheral blood mononuclear cell separation
White blood cells or peripheral blood mononuclear cells (PBMCs) should be separated. The procedure of separation of PBMCs from the collected blood is slightly different if using CPT blood collection tubes or lithium heparin tubes (check the manufacturer’s instruction). Methods include centrifugation or the use of Ficoll® plates. Once separated, the PBMCs are washed several times, centrifuged and resuspended to eliminate all other products (e.g. gammaglobulins) that are normally in human blood and could interfere with the test. Next, PBMCs are counted because 250 000 cells (±50 000) are needed for each well of the test cells.
Step 3. Pre-coated well
After the PBMCs have been separated from the whole blood specimen, a standard number of PBMCs are added to four pre-coated wells supplied by the manufacturer: one containing nil (negative control), one containing phytohemagglutinin (positive control) and two containing M. tuberculosis–specific antigens. There is no need for a standard curve with this test.
Step 4. Incubation overnight
Enzyme-linked immunosorbent spot (ELISPOT) substrate is added to each well, along with 250 000 PBMCs. These wells are then incubated at 37 °C; the incubator needs to have 5% CO2 and humidification.
Step 5. Wash, develop and dry plate
After the incubation, phosphate buffered saline (PBS) solution is used to wash the plate and is then discarded.
This washing should be repeated four times. After the fourth wash, conjugate reagent is added and incubated for 1 hour. The conjugate is then discarded and washing with PBS solution four times is repeated. Substrates are added and incubated for 7 minutes, then washing with PBS is again repeated four times. Finally, the plate is left to dry for 4 hours at 37 °C or 16 hours at room temperature.
Step 6. Count spots and interpret test results
Fig. A2.3.1. T-SPOT.TB workflow – sample preparation and testing procedures
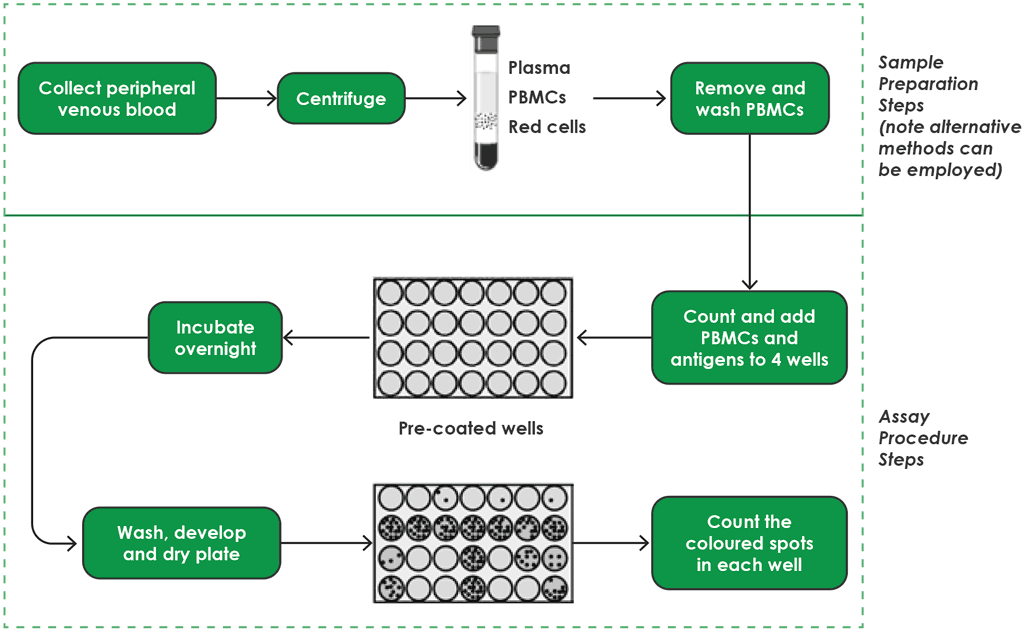
PBMC: peripheral blood mononuclear cell.
Source: product insert (4).
Materials supplied by manufacturer
Materials provided by the manufacturer comprise a microtiter plate, panel A (which comprises early secretory antigenic 6 kDa [ESAT-6] antigens), panel B (which comprises culture filtrate protein 10 [CFP10] antigens) and a positive control (which contains phytohemagglutinin, conjugate reagent and substrate solution). These components of the kit should be stored at 2–8 °C, and prolonged exposure of the substrate solution to light should be avoided.
Equipment needed in the laboratory
The following equipment is required in the laboratory for the T-SPOT.TB:
- biohazard level 2 cabinet (recommended for reagent and sample preparation);
- timer;
- equipment to count the PBMCs (ideally, an automated counter such as a haematology analyser or dedicated cell counter);
- humidified incubator capable of maintaining a temperature of 37 °C, ±1 °C with 5% CO2);
- microtiter plate washer or equipment to manually wash plates; and
- equipment for reading the spots on the plates (e.g. a microscope, digital microscope, magnifying glass or plate imager).
Materials needed
The following materials are required for the T-SPOT.TB:
- blood collection tubes (e.g. Vacutainer CPT or lithium heparin tubes);
- pipettes and sterile pipette tips; and
- reagents needed to count the PBMCs.
Personnel needed
A trained phlebotomist is required initially to draw blood samples and handle the samples. Laboratory technician time is needed for initial preparation (i.e. for the PBMC separation and counting, and setting up the wells), then significant technician time is needed after incubation for the washing and addition of reagents; finally, technician time is needed for counting the spots.
Training, supervision and QC
Personnel should be trained in the assay procedure and need to understand the instructions for use before performing the assay. Personnel require ongoing monitoring and evaluation for QC
Precautions
Laboratory technicians should always wear protective clothing (including a laboratory coat, disposable gloves and eye goggles) when working with the chemical reagents needed for this test. The T-SPOT.TB is intended for in vitro use only. Failure to adhere to the manufacturer’s instructions may lead to incorrect results.
Interpretation of T-SPOT.TB
Fig. A2.3.2 shows a flow diagram for the algorithm for interpretation of the results of the T-SPOT.TB.
Fig. A2.3.2. Algorithm flow diagram for interpretation of T-SPOT.TB
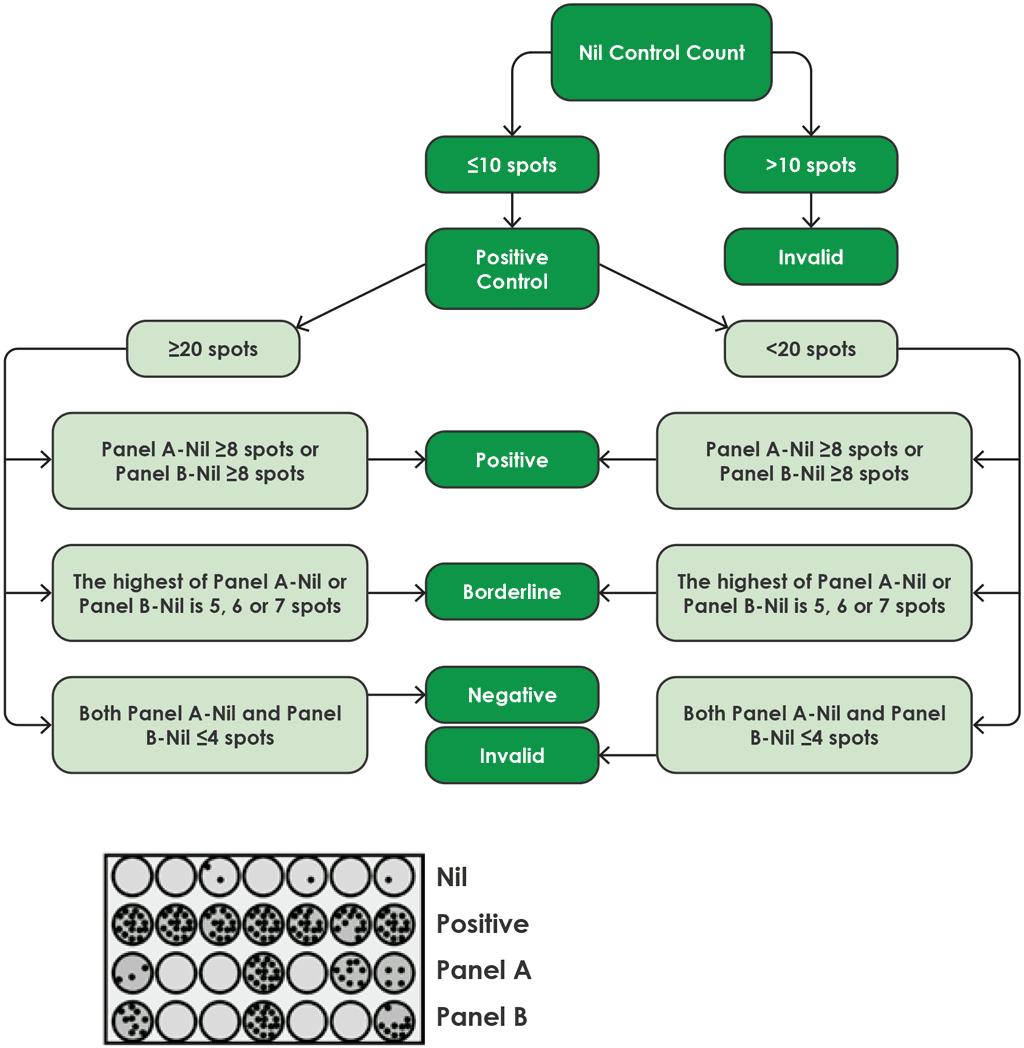
Source: product insert (4)
Results for the T-SPOT.TB test are interpreted by subtracting the spot count in the nil control well from the spot count in each of the panels, according to the following algorithm provided by the manufacturer (4):
- The test result is positive if (Panel A-Nil) and/or (Panel B-Nil) ≥8 spots (Table A2.3.1, below).
- The test result is negative if both (Panel A-Nil) and (Panel B-Nil) ≤4 spots (includes values of zero) (Table A2.3.2, below).
- The test result should be considered borderline if the highest of the spot counts from Panel A or Panel B is such that the (Panel minus Nil) spot count is 5, 6 or 7 spots. In such cases, re-testing by collecting another patient specimen is recommended (Table A2.3.3, below).
- If the test result is still borderline, then other diagnostic tests or epidemiological information should be used to help determine the patient’s TB infection status.
T-cells releasing interferon-gamma are counted using an ELISPOT assay. The test result is reported as the number of interferon-gamma producing T-cells (i.e. spot-forming cells), and the result is classified by the manufacturer as positive, negative or borderline/equivocal.
Table A2.3.1. Positive T-SPOT.TB interpretation: either (Panel A-Nil) or (Panel B-Nil) ≥8 spots
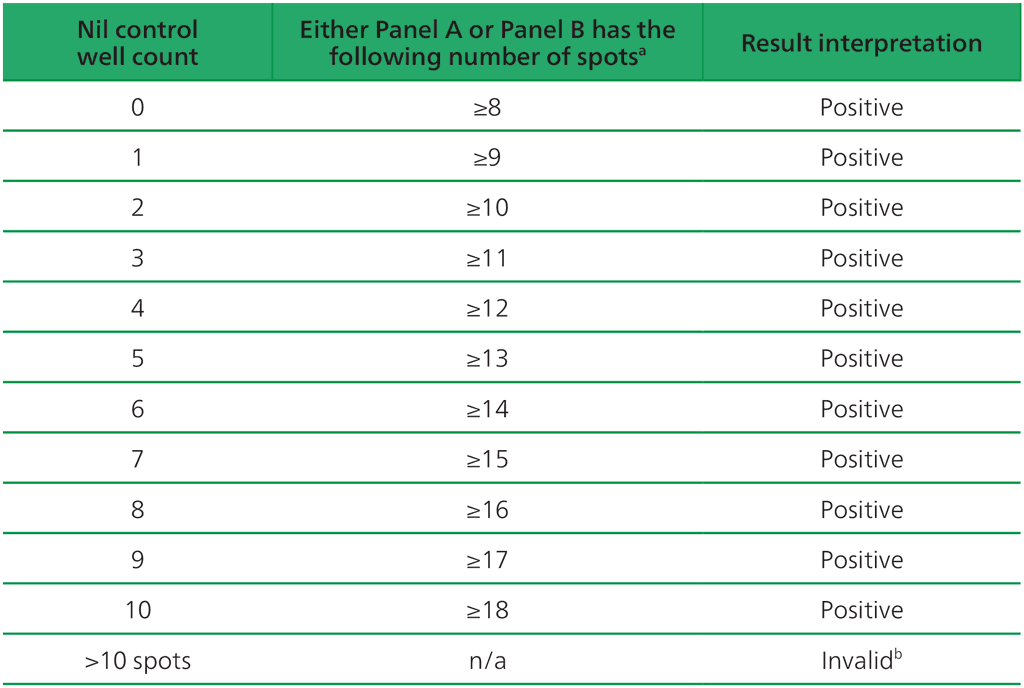
ᵃ The highest Panel-Nil spot count is to be used to determine the test outcome.
ᵇ In the case of invalid results, these should be reported as “Invalid” and it is recommended to collect another sample and re-test the individual.
Table A2.3.2. Negative interpretation: both (Panel A-Nil) or (Panel B-Nil) ≤4 spots
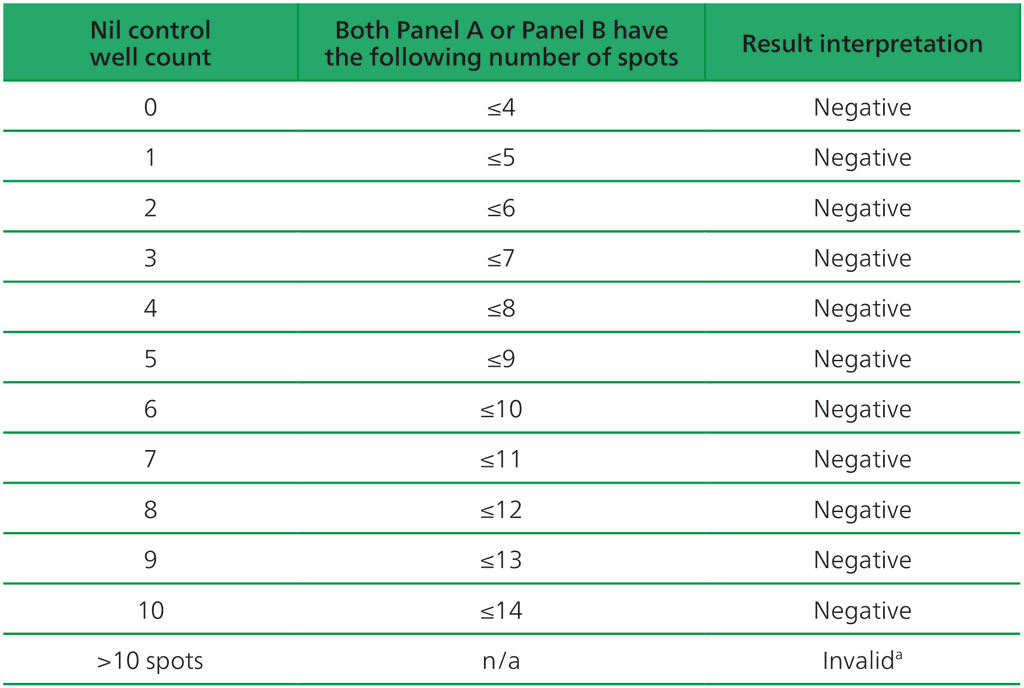
ᵃIn the case of invalid results, these should be reported as “Invalid” and it is recommended to collect another sample and re-test the individual.
Table A2.3.3. Borderline (equivocal) interpretation: the highest of (Panel A-Nil) or (Panel B-Nil) is 5, 6 or 7 spots
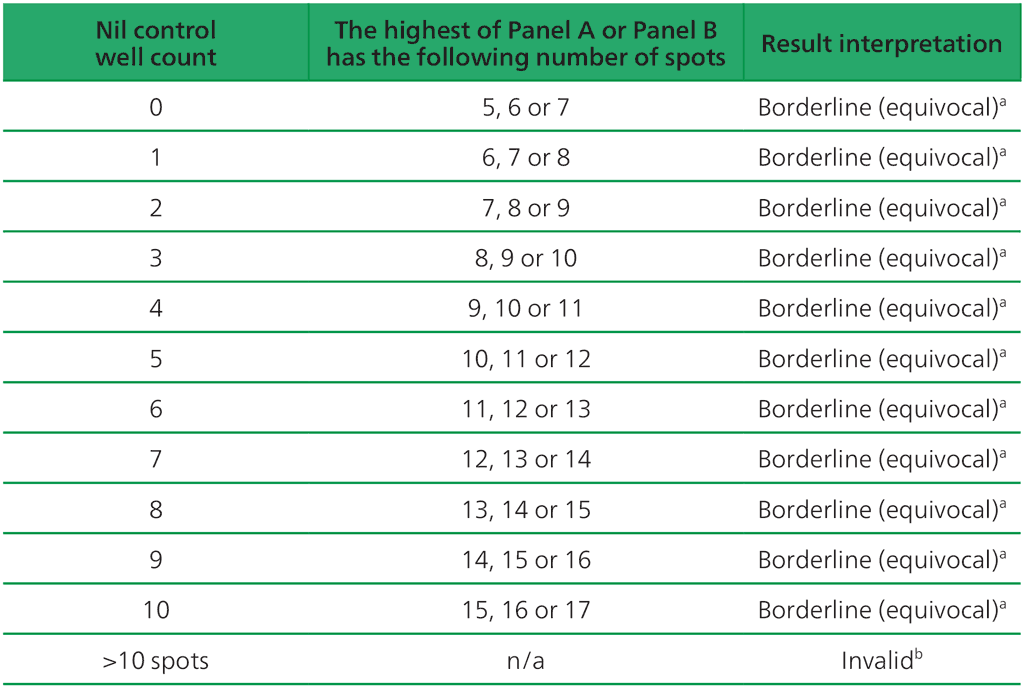
ᵃ Results where the highest of the Panel A or Panel B spot count is such that the (Panel minus Nil) spot count is 5, 6 or 7 spots should be considered borderline (equivocal) and re-testing by collecting another patient specimen is recommended.
ᵇIn the case of invalid results, these should be reported as “Invalid” and it is recommended to collect another sample and re-test the individual.
 Retour
Retour