Liens transversaux de livre pour Annex 3. GRADE summary of evidence tables
Older terminology used in the context of TPT, such as latent TB infection (LTBI) and active TB, has been retained in the original text of the tables.
PICO 1: What is the prevalence of TB infection, the risk of progression to TB disease and the cumulative prevalence of TB disease among household contacts without HIV in different age groups in high TB incidence countries?
Is the prevalence of TB disease and TB infection higher among household contacts without HIV than in the general population in different age groups in high TB incidence countries?
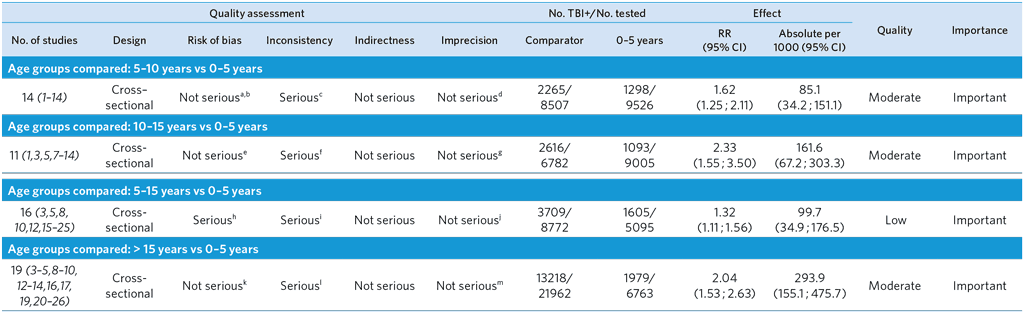
a Potential selection bias in (2), as only 69% of participants were household contacts.
b Potential misclassification: Eight studies (3–5,7,10,11,13,14) did not indicate whether household contacts with active TB were excluded from the analysis or did not provide sufficient data to calculate the number of household contacts with active TB per age stratum.
c High heterogeneity among studies (I2 = 94%) probably due to difference in background TB incidence. Risk ratios of two studies (1,5) showed opposite effect.
d Small sample size in (5) (n < 50).
e Potential misclassification: Seven studies (3,5,6,10,11,13,14) did not indicate whether household contacts with active TB were excluded from the analysis or did not provide sufficient data to calculate the number of household contacts with active TB per age stratum.
f High heterogeneity among studies (I2 = 97%) probably due to differences in background TB incidence. Risk ratio of one study (5) showed opposite effect.
g Wide confidence interval of pooled risk ratio. Small sample sizes in (5) (n < 50) and (12) (n < 100).
h Potential selection bias in (15), as only 89% of participants were household contacts.
i High heterogeneity among studies (I2 = 93%) probably due to differences in background TB incidence. Risk ratios in three studies showed opposite effects (5,19,21).
j Small sample size in (5) and (18) (n < 50).
k Potential misclassification: Ten studies (3–5,10,13,14,20,21,23,26) did not indicate whether household contacts with active TB were excluded from the analysis or did not provide sufficient data to calculate the number of household contacts with active TB per age stratum.
l High heterogeneity among studies (I2 = 98%) probably due to differences in background TB incidence.
m Small sample sizes in (5) and (26) (n < 100).
Development of TB disease in household contacts with TB infection in high TB incidence countries
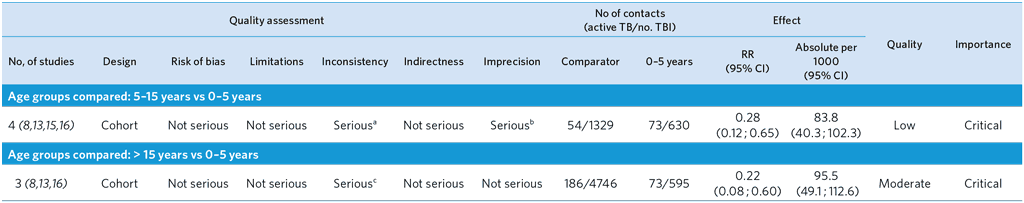
Because of the small number of studies in the other categories, only data from studies with a follow-up of 1–2 years in high TB incidence countries are presented in the table.
a Serious inconsistencies due to heterogeneity (I2 = 71%): One study showed an increased risk in the age group 5–15 years. This was not observed in the other studies.
b Small number of events.
c High heterogeneity among studies probably due to differences in background TB incidence and methods used to diagnose active TB (I2 = 89.3%).
Cumulative prevalence of TB disease in household contacts irrespective of baseline TB infection status in high TB incidence countries
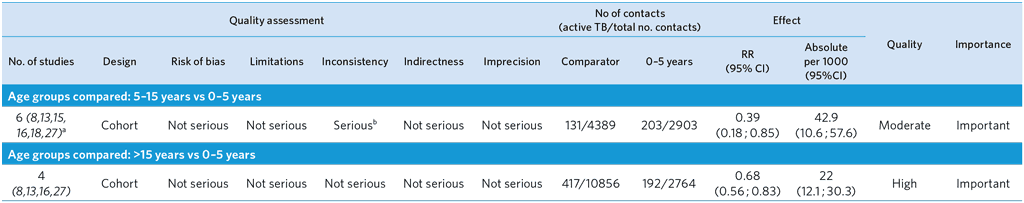
Owing to the small number of studies in the other categories, only data from studies with a follow-up of 1–2 years in high TB incidence countries are presented in the table.
a One outlier (28) was excluded because of uncertainty about the cases included (co-prevalent vs incident cases).
b High heterogeneity among studies (I2 = 87.6%), probably due to the difference in background TB incidence.
TB disease in household contacts with TB infection and in the general population in high TB incidence countries (12 months)
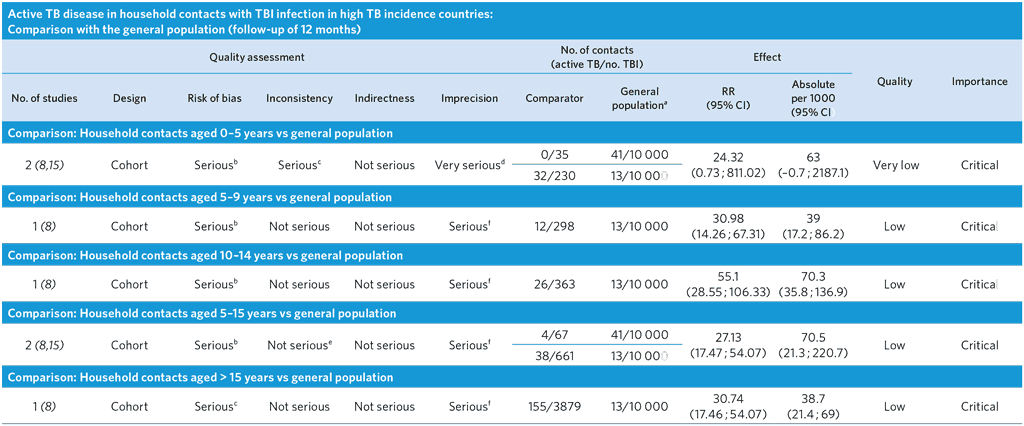
a TBI does not apply to the general population.
b Ascertainment bias highly likely, as TB cases in the general population detected passively, while TB cases in contacts detected actively. As a result, the relative and absolute risks might be overestimated. The composition of the general and the study population differed (general population of all ages versus a specific age group).
c High heterogeneity among studies (I2 = 83.9%), probably due to differences in background TB incidence.
d Serious imprecision with a wide confidence interval for the effect estimates, probably due to small study size and number of outcome events.
e I2 = 72.5%, indicating moderate heterogeneity, probably due to differences in background TB prevalence; however, there is a trend across age groups and studies.
f Few events and wide CI.
TB disease in household contacts with TB infection compared with general population in high TB incidence countries (24 months)
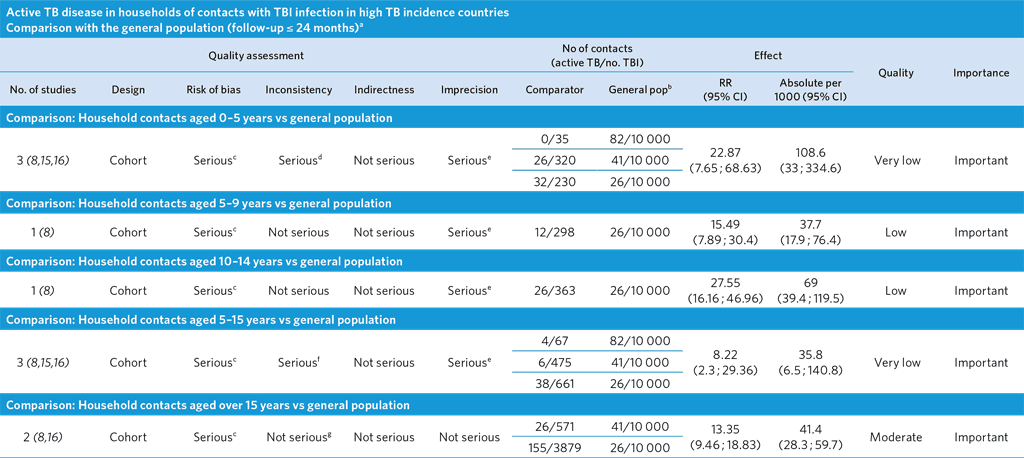
a These comparisons included studies with a maximum follow-up of 24 months; therefore, TB incidence in the general population was multiplied by a factor of 2 to estimate the number of cases occurring during 24 months.
b TBI does not apply to the general population.
c Ascertainment bias highly likely: TB cases in the general population detected passively, while TB cases in the contacts detected actively. As a result, relative and absolute risks might be overestimated. The composition of the general and study populations differs (general population of all ages versus a specific age group). TB incidence in the population was estimated by multiplying the yearly notification rate by a factor of 2.
d High heterogeneity between studies probably due to difference in background TB incidence (I2 = 84.4%).
e Few events and wide CI.
f I2 = 88.1%, indicating high heterogeneity probably due to difference in background TB prevalence; however, there is a trend across age groups and studies.
g I2 = 16%.
TB disease in household contacts irrespective of TB infection status compared with general population in high TB incidence countries (12 months)
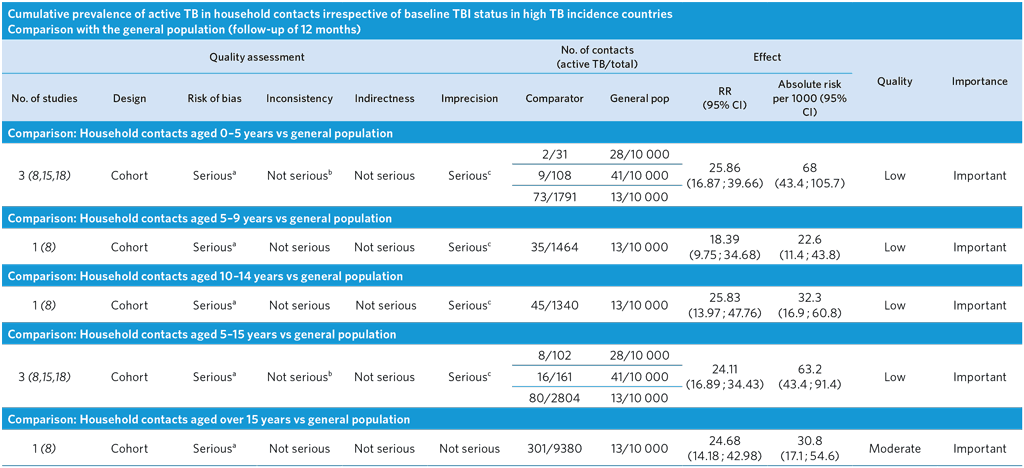
a Ascertainment bias highly likely, as TB cases in the general population detected passively, while TB cases in the contacts detected actively. As a result, relative and absolute risks might be overestimated. The composition of the general and study populations differs (general population of all ages versus a specific age group).
b I2 = 0%.
c Few events and wide CI.
TB disease in household contacts irrespective of TB infection status compared with general population in high TB incidence countries (24 months)
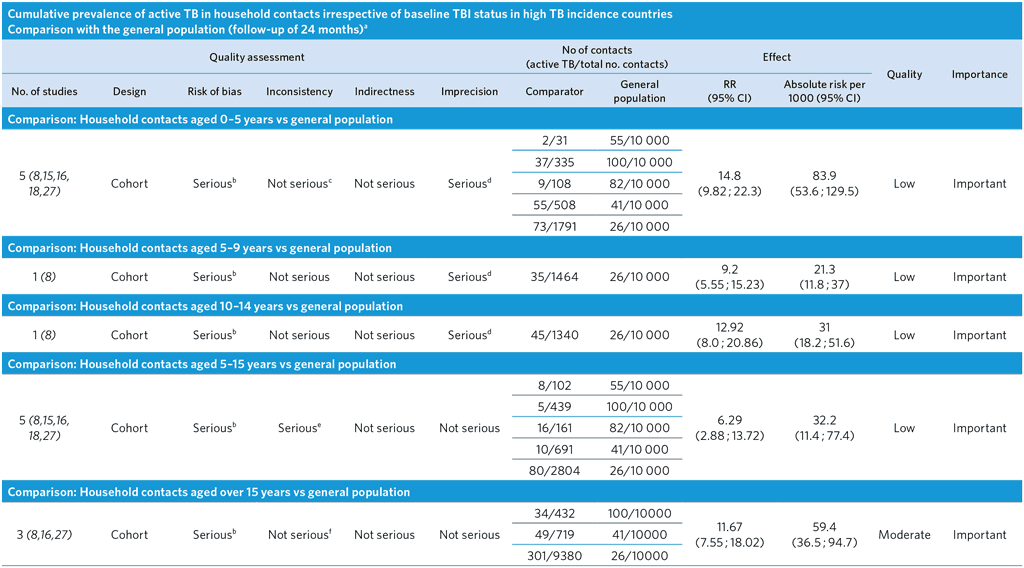
a These comparisons are based on studies with a maximum follow-up of 24 months; therefore, TB incidence in the general population was multiplied by a factor of 2 to estimate the number of cases occurring during 24 months.
b Ascertainment bias highly likely, as TB cases in the general population detected passively, while TB cases in the contacts detected actively. As a result, the relative and absolute risks might be overestimated. The composition of the general and study populations differs (general population of all ages versus a specific age group). TB incidence in the population was estimated by multiplying the yearly notification rate by a factor of 2.
c Moderate heterogeneity among studies (I2 = 67.1%) probably due to differences in background TB incidence.
d Few events and wide CI.
e High heterogeneity among studies (I2 = 87.5%) probably due to differences in background TB incidence.
f Moderate heterogeneity among studies (I2 = 72.5%) probably due to differences in background TB incidence.
PICO 2: What is the accuracy of WHO symptomatic screening to exclude TB disease in individuals with HIV on antiretroviral treatment (ART)?
Four-symptom screening plus chest radiographic findings to exclude TB disease in individuals with HIV
Population: Adults and adolescents with HIV on ART
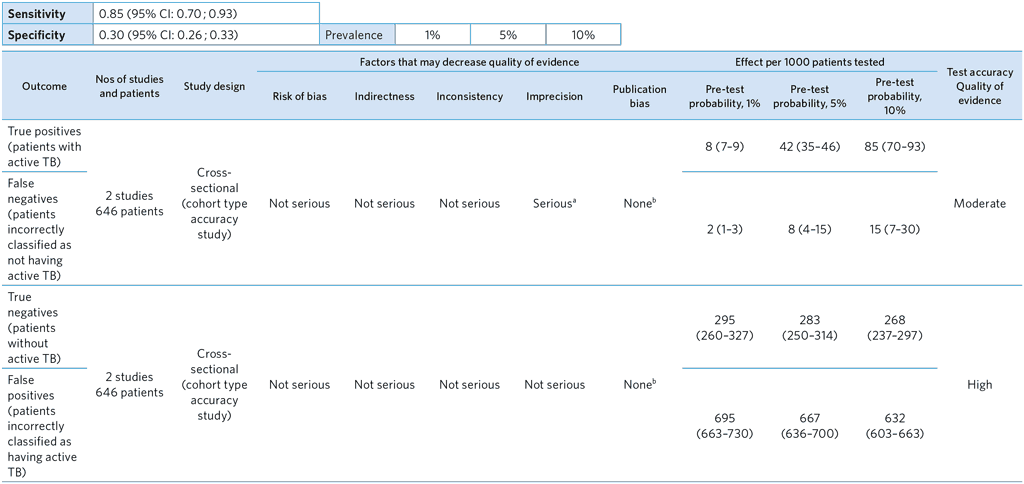
From references (29,30)
a Imprecise estimate for sensitivity. Downgraded by one.
b The possibility of publication bias is not excluded, but it was not considered of sufficient concern to downgrade.
PICO 3: What is the accuracy of symptomatic screening and/or chest x-ray to exclude TB disease in contacts of people with pulmonary TB without HIV in high TB incidence countries?
Chest radiographic findings for exclusion of TB disease in contacts of people with TB without HIV in high TB incidence countries
Index test: Chest X-ray. Any abnormality | Reference test: Sputum culture and/or smear
Place of testing: Triage
Test–treatment pathway: Chest X-ray positive ➞ confirmatory test (mycobacterial culture or GeneXpert) ➞ anti-TB chemotherapy (6–9 months’ antibiotics)
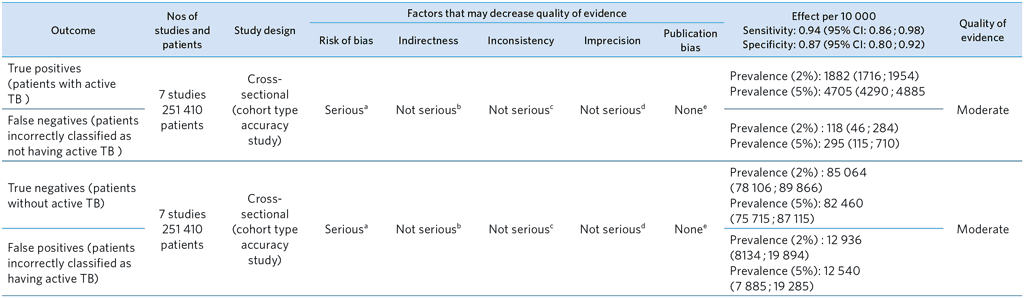
From references (31–37)
a Limitations in study design (see QUADAS-2): High risk of selection bias in one study (31). In all studies, less than half of participants received the reference standard; accuracy was calculated under the assumption that those who did not receive the reference standard were culture and/or smear negative (no active TB).
b Indirectness (see QUADAS-2): Some concern about applicability of reference standard in 2 studies – no downgrading.
c Inconsistency: Little heterogeneity for sensitivity and specificity (based on visual inspection of CIs).
d Imprecision: Precise estimates for sensitivity and specificity.
e Publication bias: Not applicable (the evidence base for publication bias in studies of diagnostic test accuracy is very limited).
Any symptom for exclusion of TB disease in contacts of people with TB without HIV in high TB incidence countries
Index text: Any symptom | Reference test: Sputum culture and/or smear
Place of testing: Triage
Test–treatment pathway: Symptom positive ➞ confirmatory test (mycobacterial culture or GeneXpert) ➞ anti-TB chemotherapy (6–9 months’ antibiotics)
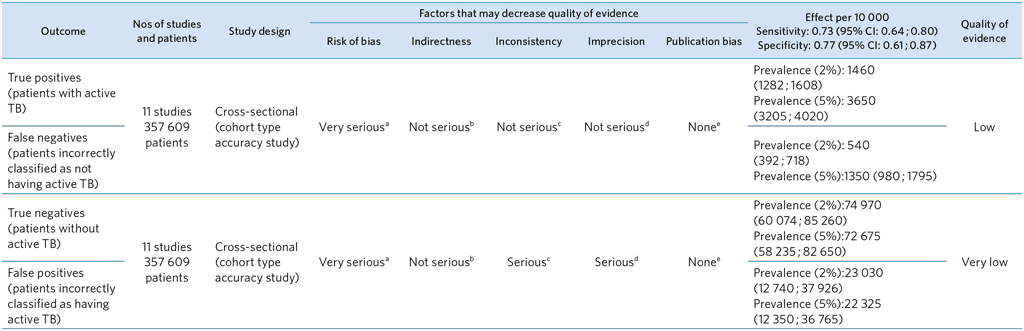
From references (31–34,36,38–43)
a Limitations in study design (see QUADAS-2): high risk of selection bias in 1 study (den Boon, 2006) and in two studies unclear risk of bias for the reference standard. In 9 of the 11 studies less than half the participants received the reference standard; accuracy was calculated under the assumption that those who did not receive the reference standard were culture and/or smear negative (no active TB) .
b Indirectness (see QUADAS-2): No major concern about applicability.
c Inconsistency: Moderate heterogeneity for sensitivity and significant heterogeneity for specificity (based on visual inspection of CIs) – downgrading on specificity.
d Imprecision: Precise estimates for sensitivity and imprecise estimate for specificity.
e Publication bias: Not applicable (the evidence base for assessing publication bias in studies of diagnostic test accuracy is very limited.
PICO 4: Could interferon-γ release assays be used as an alternative to tuberculin skin tests to identify individuals at greatest risk of progression from TB infection to TB disease in high TB incidence settings?
TST or IGRA for identifying individuals at greatest risk of progression to TB disease
Head-to head-evaluations of TST and IGRA (N = 5)
Review question: Among people at high risk of TBI who are not treated with TB preventive therapy, which test (e.g. TST or IGRA), when positive, can best identify individuals most at risk of progression?
Outcome: Predictive utility of the TST vs commercial IGRAs for progression to active TB Patients/population: Longitudinal studies of adults and children without active TB at baseline not treated with preventive therapy
Setting: Community cohorts, individuals attending outpatient clinics (e.g. people living with HIV), individuals participating in RCTs, household contacts; all in high-incidence countries
Index test: TST (RT23 purified protein derivative or purified protein derivative S) and/or commercial blood-based IGRAs (QuantiFERON®-TB Gold In-Tube and T-SPOT®.TB)
Importance: Longitudinal studies on the predictive value of a positive IGRA are still emerging in TB high-incidence countries (≥ 100/100 000). It is important to assess whether IGRA can be used as a replacement for the widely used TST.
Reference standard: All diagnoses of incident active TB (microbiologically confirmed or not)
Studies: Any longitudinal study design (e.g. prospective or retrospective cohort), in TB high-incidence countries, regardless of immunological status (e.g. HIV-infected or not) or BCG status. Average follow-up should be ≥ 1 year, but can be either active or passive.
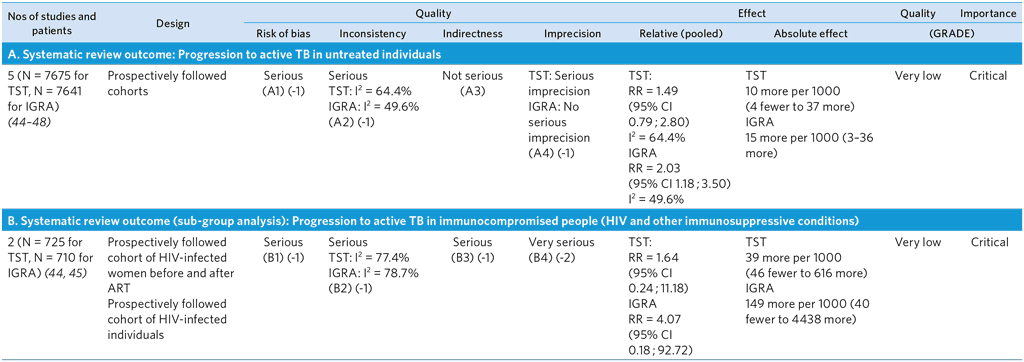
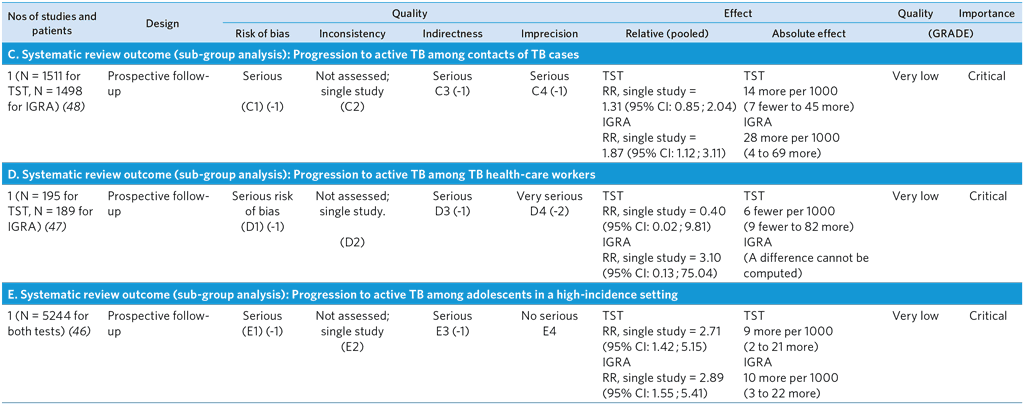
Notes on GRADE summary table
Overall quality:
All studies start with one point taken off because none were RCTs. The lowest quality score achievable is 1 out of 4; no minus scores are given.
Quality assessment: Based on the relative effect measure (RR or IRR) for both TST and IGRA. Studies not marked down if estimates for both tests score high on a specific GRADE quality item.
Other study quality considerations: Newcastle-Ottawa Scale quality items were considered when assessing the risk of bias. One point is docked if at least one concern is present.
A1: Risk of bias is possible. Issues in the studies include selection bias, risk of incorporation bias, ascertainment and publication bias. Methods for ascertaining TB included microbiological methods, but not all incident TB cases had a definite culture-confirmed diagnosis of TB. Publication bias not formally assessed, but expected to be likely. Several large prospective studies are ongoing and/or unpublished; their results were not included in this analysis; however, addition of their results is not expected to change the overall conclusions of this review.
A2: Serious unexplained inconsistency of RR estimate for TST. Points taken off if serious inconsistency identified in either estimate.
A3: Although the number of studies included is small, they involve a range of populations, including adults and children, immunocompromised people and TB contacts, providing direct evidence for these groups.
A4: Serious imprecision of RR estimate for TST. Lower limit of 95% CI indicates lack of predictive utility. Points docked if serious imprecision identified in either estimate.
B1: Risk of bias is possible. Issues include selection bias, risk of incorporation bias, ascertainment and publication bias. Incorporation bias could not be ruled out in the cohort that included antepartum and postpartum women because information was not available; moreover, there is concern about selection. The ART cohort study reported reference standards that do not account for index tests; however, assessors were not blinded to baseline TST results that were recorded in patient records. Methods for ascertaining TB included microbiological methods, but not all incident TB cases had a definitive diagnosis of TB. Publication bias not formally assessed but expected to be likely. Several large prospective studies are ongoing and/or are unpublished; their results were not included in this analysis; however, addition of results is not expected to change the overall conclusions of this review.
B2: Serious unexplained inconsistency in RR estimates for both TST and IGRA.
B3: This pooled estimate is based on only two studies: one study of HIV-infected people on ART with a median CD4+ approximately 250, and one on HIV-infected antepartum and postpartum women. No direct evidence for treatment-naïve patients and/or HIV-infected patients with high CD4 counts or other sub-populations of HIV-infected individuals (e.g. children).
B4: Very serious imprecision of RR estimates for both TST and IGRA. CIs are wide and indicate both significant predictive performance and lack of predictive utility. Studies had few events.
C1: Risk of bias is possible. Issues include selection bias, risk of incorporation bias (no information) and publication bias. Publication bias not formally assessed, but expected to be likely. Several large prospective studies are ongoing and/or are unpublished; their results were not included in this analysis; however, addition of results is not expected to change the overall conclusions of this review.
C2: Inconsistency not assessed.
C3: This single study comprises household case contacts in a high-incidence country. No direct evidence for other subpopulations of case contacts.
C4: Serious imprecision of TST effect estimates. Lower limit of 95% CI indicates lack of predictive utility.
D1: Risk of bias is possible. Issues include selection bias, lack of use of microbiological tools in methods to ascertain TB, incorporation bias and publication bias. Publication bias not formally assessed, but expected to be likely. Several large prospective studies are ongoing and/or are unpublished; their results were not included in this analysis; however, addition of results is not expected to change the overall conclusions of this review.
D2: Inconsistency not assessed.
D3: This single study comprises health-care workers at a primary health care clinic. No direct evidence for other subpopulations of health-care workers or all settings of health care.
D4: Very serious imprecision of IGRA and TST effect estimates; CIs are wide and indicate both significant predictive performance and lack of predictive utility.
E1: Risk of bias is possible. Issues include selection bias, incorporation of index tests in methods to ascertain incident TB and publication bias. Publication bias not formally assessed, but expected to be likely. Several large prospective studies are ongoing and/or are unpublished; their results were not included in this analysis; however, addition of results is not expected to change the overall conclusions of this review.
E2: Inconsistency not assessed.
E3: This single study comprises adolescents in a high-incidence setting. No direct evidence for other subpopulations of children or adolescents.
E4: No serious imprecision: Few events with large sample size.
PICO 5: Should 3-month daily rifampicin plus isoniazid (3RH) be offered as a preventive treatment option for children and adolescents <15 years of age as an alternative to 6 or 9 months' isoniazid (INH) monotherapy in high TB incidence countries?
3-month daily rifampicin and isoniazid in children and adolescents < 15 years
Overall quality: low
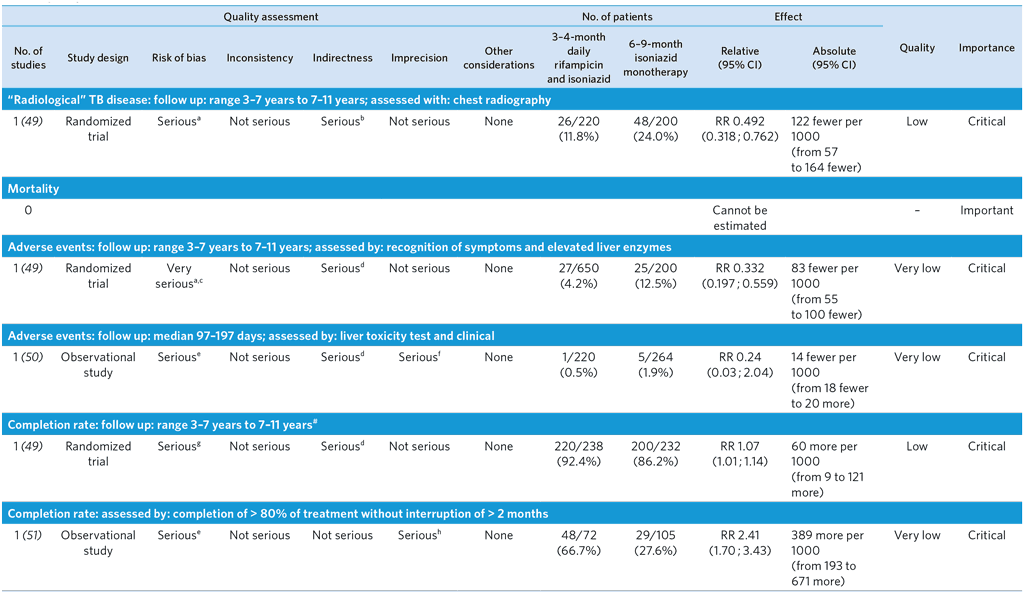
From references (49–51)
a Although there was a risk of selection bias, the characteristics of the two groups were similar. Patients with poor compliance were not included in the analysis of treatment outcomes. Downgraded by one level.
b There was no clinical disease. The outcome reported was new radiographic findings suggesting possible active disease. No data compared with 6H. Downgraded by one level.
c A high risk of detection bias due to lack of blinding. The RH group included participants enrolled during the second period, whose characteristics were different; they were not randomized between the RH group and the 9H group. Downgraded by two levels.
d No data compared with 6H. Downgraded by one level.
e Risk of bias due to poor comparability of the two groups. Downgraded by one level.
f Low event rate and wide 95% CI. Downgraded by one level.
g Lack of blinding. Medication adherence test was performed at home by parents. Although there was a risk of selection bias, the characteristics of the two groups were similar. Downgraded by one level.
h Wide 95% CI. Downgraded by one level.
# The study reported adherence rates; compliance was considered to be poor if no medication was detected in urine strips or if patients did not return for follow-up visits or were lost to follow-up. Poor compliance was considered non-completion in the analysis.
PICO 6: In people of all ages at risk of TB disease, does a 4-month daily rifampicin regimen safely prevent TB disease as compared with other recommended TB preventive treatment regimens?
Overall quality: moderate
Bibliography: (see references 52–56)
Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, et al. Four Months of Rifampin or Nine Months of Isoniazid for Latent Tuberculosis in Adults. New Eng J Med. 2018 Aug 2;379(5):440–53.
Diallo T, Adjobimey M, Ruslami R, Trajman A, Sow O, Obeng Baah, J, et al. Safety and Side Effects of Rifampin versus Isoniazid in Children. N Engl J Med. 2018;379:454–463.
Menzies D, Long R, Trajman A, Dion MJ, Yang J, Al Jahdali H, et al. Adverse Events with 4 Months of Rifampin Therapy or 9 Months of Isoniazid Therapy for Latent Tuberculosis Infection: A Randomized Trial. Ann Intern Med. 2008;149(10):689–697.
Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am J Respir Crit Care Med. 2004;170(4):445–449.
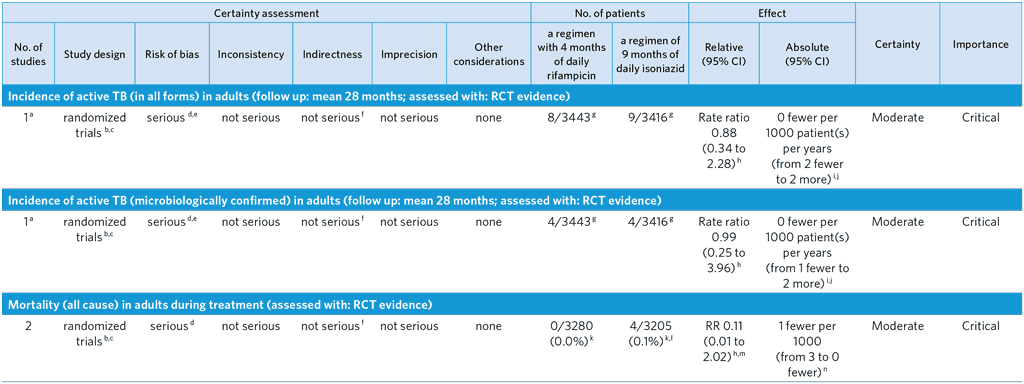
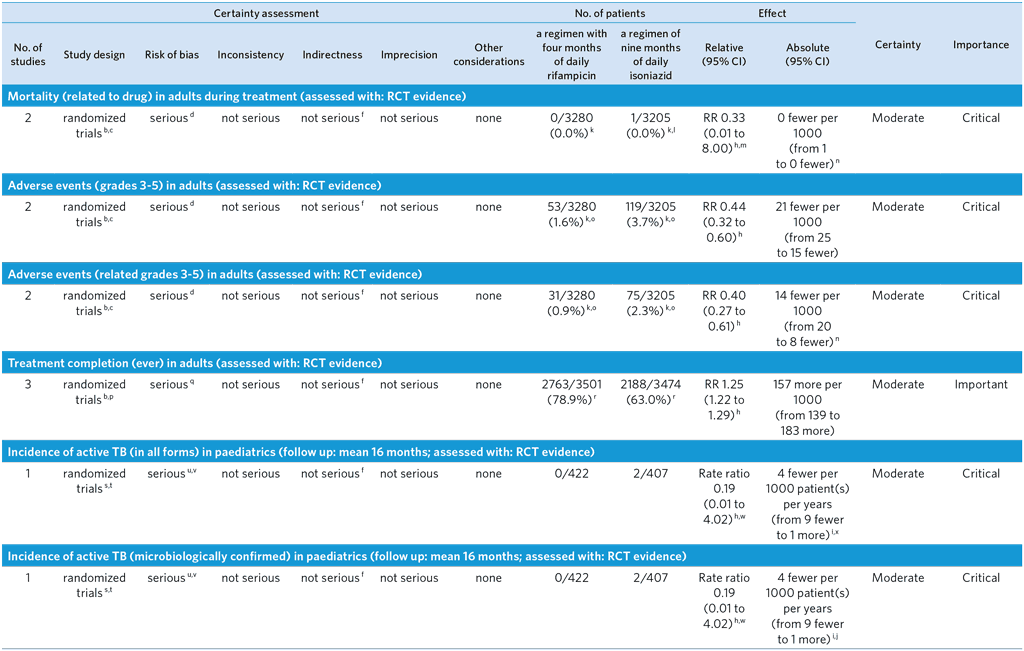
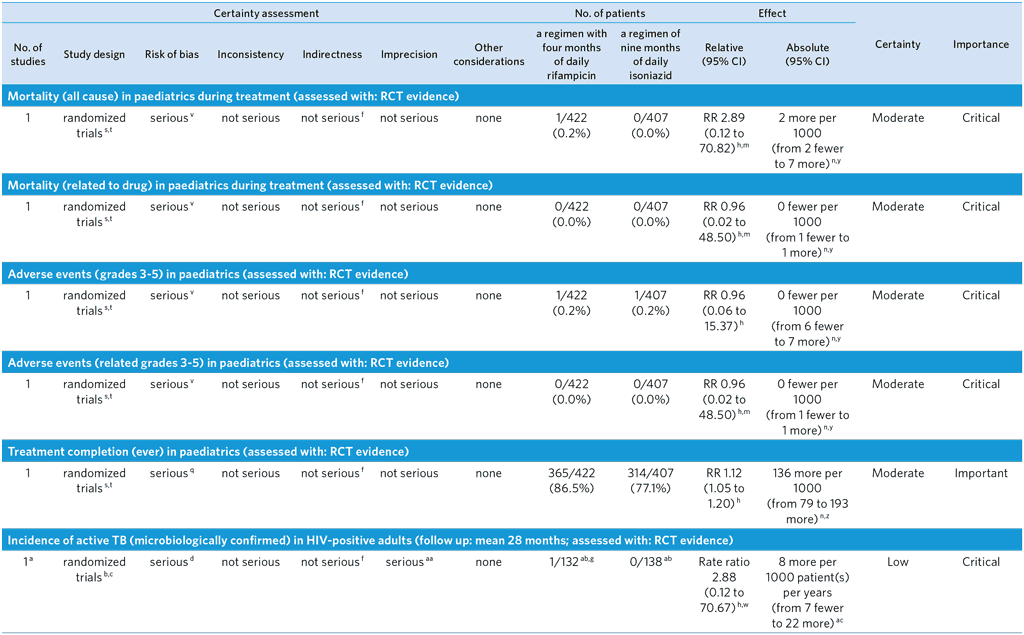
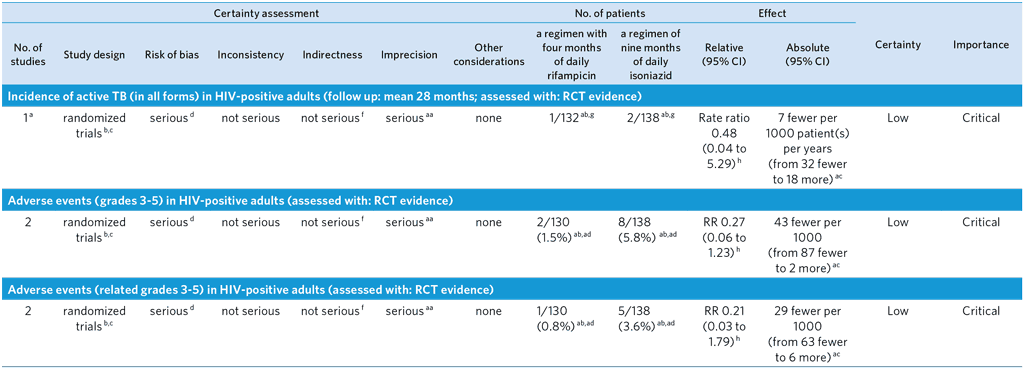
CI: Confidence interval; RR: Risk ratio
Explanations
a The GDG decided that for efficacy outcomes the pooled outcomes for phase 2 and phase 3 studies be considered one trial as the same protocol was used for both phases conducted by the same investigating team, even if the number of sites increased in the phase 3 study. Although the quality was not downgraded for this, the GDG noted that Inconsistency could not be judged given that there was only a single trial. Ideally replication by other trials would be desirable. For adverse events the studies can be considered as two separate trials.
b Phase 2 (54) and Phase 3 (52) open-label trials conducted in nine countries, assigning adults with latent tuberculosis infection to receive treatment with a 4-month regimen of daily rifampicin or a 9-month regimen of daily isoniazid. The primary outcome in the phase 2 trial was incidence of grade 3 to 5 adverse events (superiority design), with secondary outcomes of treatment completion and incidence of active tuberculosis within 28 months of randomization. The primary outcome of the phase 3 trial was microbiologically confirmed active tuberculosis within 28 months after randomization (non-inferiority design), with secondary outcomes of clinically diagnosed active tuberculosis, grade 3 to 5 adverse events, and treatment completion. Outcomes of active tuberculosis and adverse events were adjudicated by three-member, blinded, independent review panels; treatment completion based on pill counts at routine follow-up visits.
c Between the phase 2 and phase 3 trials in adults, there were no significant changes in guidelines or risk profiling of latent TB reactivation in terms of judging ‘increased risk for reactivation’. Randomization in both trials was stratified by site and centrally computer-randomized. Patients were randomized 1:1 in blocks of varying length (2 to 8) to isoniazid or rifampicin.
d Open label design but endpoints of active TB and adverse events adjudicated by three-member, independent, blinded review panels. There were 18 per protocol exclusions among those randomized to isoniazid and 19 per protocol exclusions among those randomized to rifampicin. These per protocol exclusions were due to being a household contact of a tuberculosis patient with resistance to isoniazid or rifampicin (proven post-randomization). There were nine individuals randomized to isoniazid and five individuals randomized to rifampicin who withdrew consent after randomization. The GDG decided to downgrade by one level because of the open label design possibly led to performance bias.
e Among those randomized to isoniazid and forming the modified intention-to-treat population, 260 individuals were lost to follow-up. Among those randomized to rifampicin and forming the modified intention-to-treat population, 245 individuals were lost to follow-up. In the modified intention-to-treat population, 7.4% of individuals were lost to follow-up.
f The quality was not downgraded for Indirectness, but the GDG noted that the trial compared 4R with 9H and therefore did not cover all other comparisons of the PICO, especially 6H, the most widespread standard of care in TPT. Some study sites were low TB incidence settings for which a WHO recommendation for use of 4R already exists.
g All active TB events occurred within the phase 3 trial (52).
h Unadjusted estimate.
i The rate difference was estimated by a Poisson model with the use of generalized estimating equations with a log link and the inclusion of the log of person-time as an offset. An exchangeable correlation structure with robust standard errors was used to account for the correlation of participants coming from the same household.
j Values reported as per Table 3 of (52). Values include Phase 2 results (54) as well.
k Denominators are representative of the combined safety population of phase 2 (54) and phase 3 (52) as indicated in supplemental tables S2 and S3 of the phase 3 publication. From the phase 2 trial, 396 patients receiving isoniazid and 393 patients receiving rifampicin formed the safety population; from the phase 3 trial, 2809 patients receiving isoniazid and 2887 patients receiving rifampicin formed the safety population.
l All mortality events occurred in the phase 3 trial (52).
m A zero cell correction of 0.5 has been used to calculate the risk ratio.
n The risk difference was estimated by a binomial distribution model with an identity link and generalized estimating equations. An exchangeable correlation structure and robust standard errors were used to account for correlation of patients coming from the same family. If no events occurred in one or both arms, confidence intervals were calculated based on (56).
o Among adverse events from the phase 2 trial (54), 10 patients receiving rifampicin experienced grade 3–5 adverse events which led to permanent discontinuation of the medication, of which 7 were deemed possibly/probably related to study drug; 19 patients receiving isoniazid experienced grade 3–5 adverse events which led to permanent discontinuation of the medication, of which 16 were deemed possibly/probably related to study drug. Among adverse events from the phase 3 trial (52), 43 patients receiving rifampicin experienced grade 3–5 adverse events which led to permanent discontinuation of the medication, of which 24 were deemed possibly/probably related to study drug; 100 patients receiving isoniazid experienced grade 3–5 adverse events which led to permanent discontinuation of the medication, of which 59 were deemed possibly/probably related to study drug.
p Also included is the phase 1 trial (55), a single centre, open-label randomized trial assessing superiority of 4 months of daily rifampicin to 9 months of daily isoniazid for treatment completion.
q Open label trial, unblinded assessment of compliance judged on the basis of pill counts at monthly follow-up visits.
r Numerator and denominator values are derived from the Phase 1 trial (55), Phase 2 trial (54), and Phase 3 trial (52). Treatment completion was defined as taking at least 80% of prescribed doses (i.e., at least 96 pills of rifampicin or 216 pills of isoniazid). In the phase 1 trial, 44 of 58 individuals randomized to isoniazid and 53 of 58 individuals randomized to rifampicin completed treatment. In the phase 2 trial, 254 of 427 individuals randomized to isoniazid and 328 of 420 individuals randomized to rifampicin completed treatment. In the phase 3 trial, 1890 of 2989 individuals randomized to isoniazid and 2382 of 3023 individuals randomized to rifampicin completed treatment.
s Open-label, non-inferiority trial conducted in seven countries, assigning children with latent tuberculosis infection to receive treatment with a 4-month regimen of rifampicin or a 9-month regimen of isoniazid for the incidence of grade 3 to 5 adverse events during treatment. Secondary outcomes were the incidence of microbiologically confirmed active tuberculosis within 16 months after randomization and completion of the treatment regimen. Outcomes of active TB and adverse events were adjudicated by two- or three-member, blinded, independent review panels; treatment completion based on pill counts at routine follow-up visits (53).
t Randomization in the paediatric trial was stratified by country and centrally computer-randomized. Patients were randomized 1:1 in blocks of varying length (2 to 8) to isoniazid or rifampicin. Enrollment and randomization in this trial was completely separate from the adult trials.
u Among those randomized to isoniazid and forming the modified intention-to-treat population, there were 6 individuals lost to follow-up. Among those randomized to rifampicin and forming the modified intention-to-treat population, there were 5 individuals lost to follow-up. Among all children forming the modified intention-to-treat population, 1.3% of individuals were lost to follow-up.
v Open label design but endpoints of active TB and adverse events adjudicated by two-member and three-member, respectively, independent, blinded review panels. There were 9 per protocol exclusions among those randomized to isoniazid and 6 per protocol exclusions among those randomized to rifampicin. These per protocol exclusions were due to being tuberculin skin test negative at the end of the window period (two months after exposure). GDG decided to downgrade by one level because of the open label design and because some sites were not high burden.
w A zero cell correction of 0.5 has been used to calculate the rate ratio. x Values as reported in the text of the paediatric trial (53).
y Values as reported in Table 3 of the paediatric trial (53).
z Values reported in Table 2 of the paediatric trial (53).
aa Subgroup analysis within randomized trials that involved relatively small numbers of HIV-infected patients when compared to all patients included in the trials.
ab Denominators include HIV-positive patients known at the time of randomization as reported in Supplemental Table S1 of the phase 3 adult trial (52), as well as patients diagnosed post randomization as a result of baseline assessment. This includes 130 patients and 8 patients receiving isoniazid with an HIV-diagnosis at time of randomization and post-randomization, respectively, and 125 patients and 7 patients receiving rifampicin with an HIV-diagnosis at time of randomization and post-randomization, respectively. This resulted in modified intention to treat population sizes of 132 for rifampicin and 138 for isoniazid. Among HIV-positive patients randomized to rifampicin, 2 did not receive a dose of therapy. Thus, the safety population sizes were 130 for rifampicin and 138 for isoniazid.
ac Unadjusted absolute estimate.
ad Among patients receiving rifampicin included in the safety population, 6 patients were HIV-positive in the phase 2 trial and 124 patients were HIV-positive in the phase 3 trial. All grade 3–5 adverse events among patients receiving rifampicin occurred in the phase 3 trial. Two patients experienced a grade 3–5 adverse event with rifampicin that resulted in permanent discontinuation of the study drug, only 1 was deemed possibly/probably related to the study drug. Among patients receiving isoniazid included in the safety population, 7 patients were HIV-positive in the phase 2 trial and 131 were HIV-positive in the phase 3 trial. One patient in the phase 2 trial and 7 patients in the phase 3 trial receiving isoniazid experienced a grade 3–5 adverse event resulting in permanent discontinuation of the study medication. The events were deemed possibly/probably related to the study drug for the one patient from the phase 2 trial and for 4 patients from the phase 3 trial.
PICO 7: In people of all ages at risk of TB disease, does a 1-month daily rifapentine plus isoniazid regimen safely prevent TB disease as compared with other recommended TB preventive treatment regimens?
Population: PLHIV at increased risk of active TB
Overall quality: low
Bibliography: (see reference 57)a
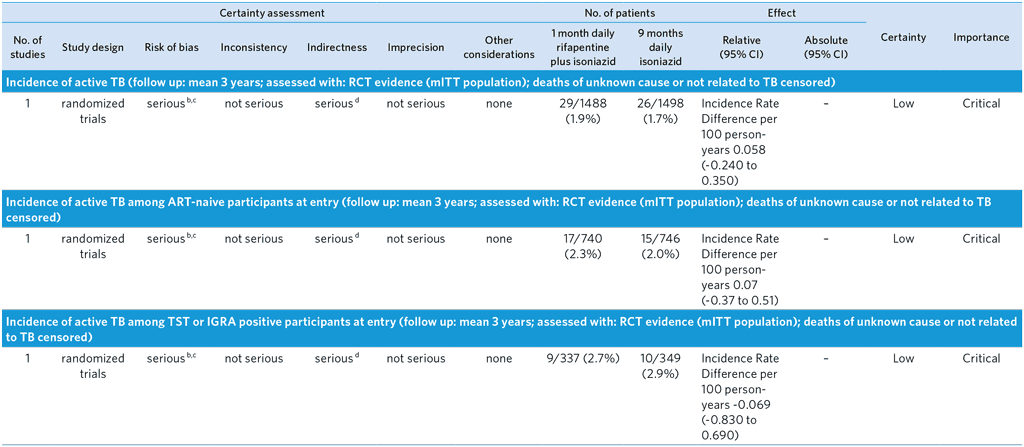
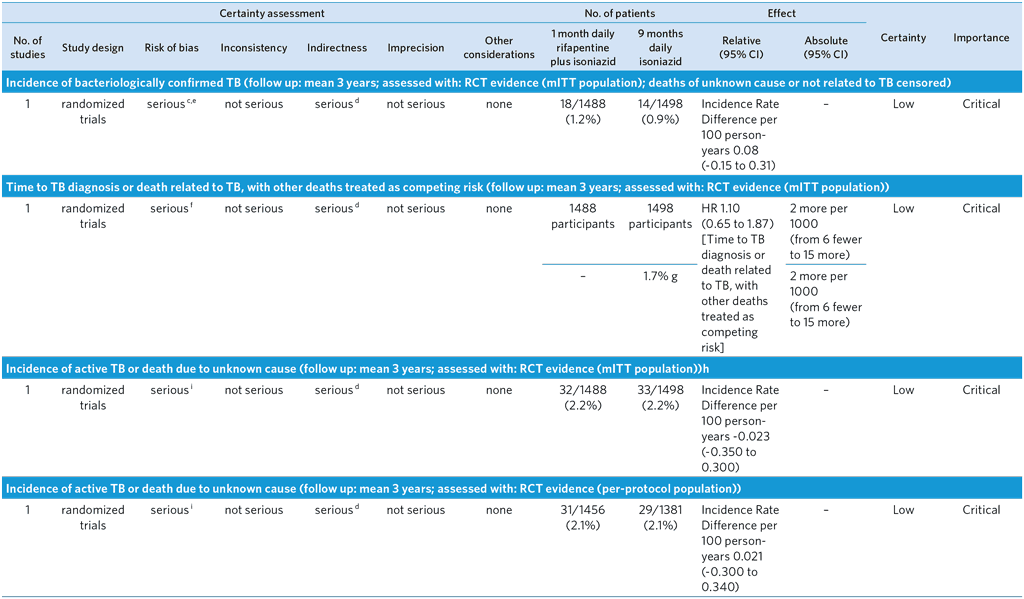
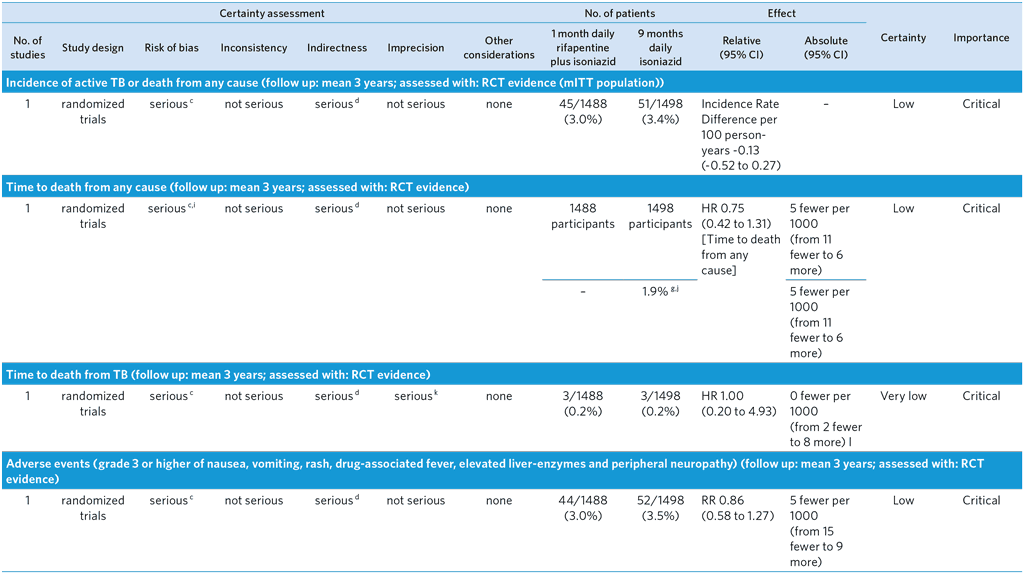
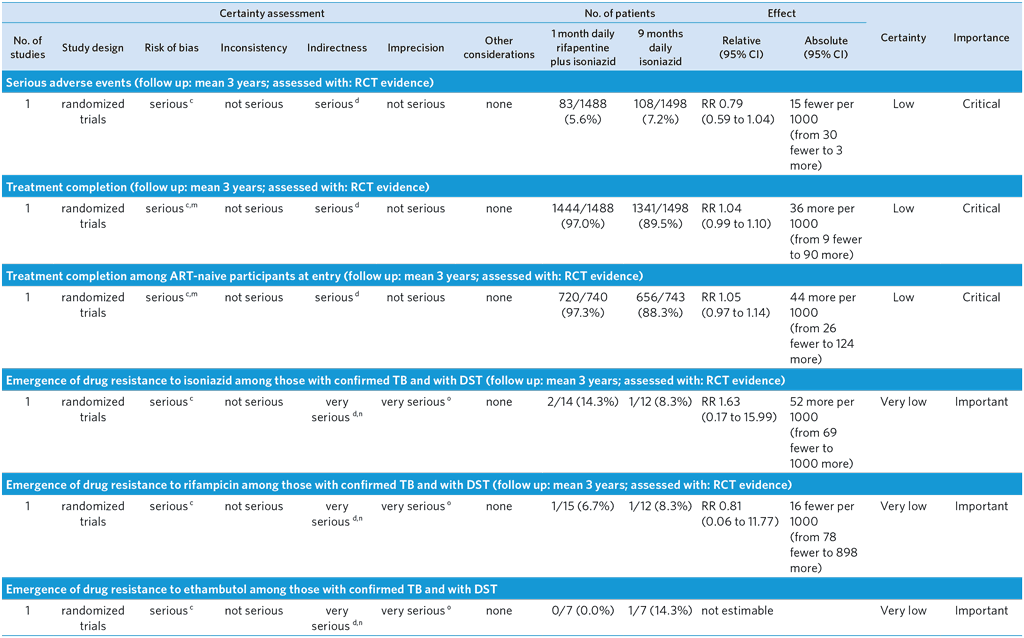

CI: Confidence interval; HR: Hazard Ratio; RR: Risk ratio
Explanations
a Randomized, open-label, phase 3 noninferiority trial comparing the efficacy and safety of a 1-month regimen of daily rifapentine plus isoniazid (1-month group) with 9 months of isoniazid alone (9-month group) in HIV-infected patients who were living in areas of high TB prevalence or who had evidence of latent TB infection. Primary end point was the first diagnosis of TB or death from TB or an unknown cause. Noninferiority would be shown if the upper limit of the 95% confidence interval for the between-group difference in the number of events per 100 person-years was less than 1.25. TBI was not confirmed in about 80% of participants. Enrolment restricted to individuals ≥13 years old who were not pregnant or breastfeeding. Overall TB incidence observed in the trial was lower than expected. The number of patients with a CD4+ <250 cells per cu mm was small, and neither inferiority nor noninferiority of the 1-month regimen was shown in this stratum.
b Unknown cause of death censored in this analysis, which may cause bias in incidence rate difference if some of these deaths were related to TB (dependent censoring)
c The GDG decided to downgrade by one level because of the open label design possibly leading to performance bias. The quality was not downgraded for Indirectness, but the GDG noted that the trial compared 1HP with 9H and therefore did not cover all other comparisons of the PICO, especially 6H, the most widespread standard of care in TPT. The GDG noted that Inconsistency could not be judged given that there was only a single trial; results from more trials would be desirable.
d Trial conducted only in PLHIV and not in all people at risk of active TB.
e Probable TB diagnoses and deaths with non-bacteriologically confirmed TB censored at the time of event
f When cause of death was determined to be unknown or not related to TB by blinded external reviewers, these were treated as a competing risk rather than endpoint. Some of these may have actually been due to TB, which may bias estimate.
g The proportion of events among controls
h Per-protocol population consisted of all participants who completed treatment, or who had died or received a TB diagnosis while they were receiving treatment.
i Deaths were reviewed by blinded external reviewers. Unknown causes of death were included as an endpoint, but misclassification of cause of death may bias estimate
j There were 21 deaths in the 1-month arm, 3 related to TB. There were 28 deaths in the 9-month arm, 3 related to TB.
k Small number of events
l Incidence rate difference per 100 person-years of 0.00 (-0.10 to 0.10)
m Assessed via participant self-report at clinic visits
n Resistance may be non-emergent and coming from infecting strain
o Small sample of bacteriologically confirmed TB who had drug susceptibility test results
PICO 8: Should 3-month weekly rifapentine and isoniazid be offered as an alternative regimen to isoniazid monotherapy for treatment of TB infection in high TB incidence countries?
3-month weekly rifapentine plus isoniazid or daily isoniazid monotherapy for TBI treatment in adults with HIV
Population: Adults with HIV
Comparison: 6 or 9 months of isoniazid monotherapy
Overall quality: high
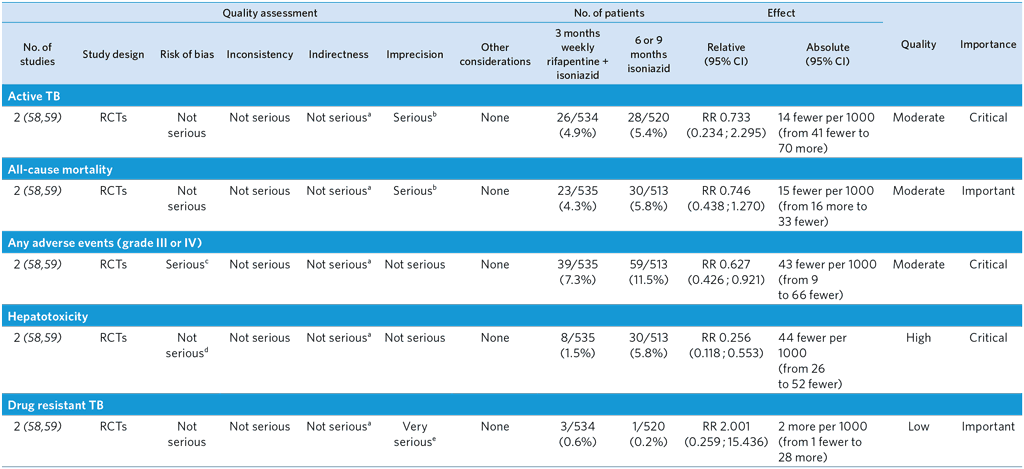
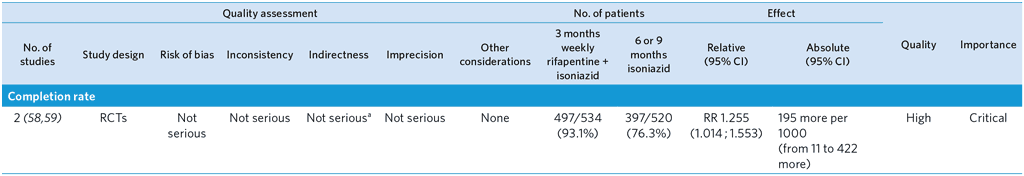
a Although one of the trials was conducted in low TB incidence countries, this is unlikely to affect the relative effect of rifapentine + isoniazid compared with isoniazid monotherapy. Not downgraded.
b 95% CIs of both relative and absolute effect include appreciable benefit and harm with 3HP.
c Both trials were open-label, which may have introduced bias in ascertainment of adverse events.
d Although the trials were open-label, this is unlikely to affect detection of hepatotoxicity, which is usually done by objective measurement (i.e. blood tests.). Not downgraded.
e Very low event rates. Upper limit of 95% CI of both relative and absolute effect include appreciable harm with 3HP. Downgraded by two levels.
3-month weekly rifapentine plus isoniazid or daily isoniazid monotherapy for treatment of TB infection in adults without HIV
Population: Adults without HIV
Comparison: 6 or 9 months of isoniazid monotherapy
Overall quality: moderate
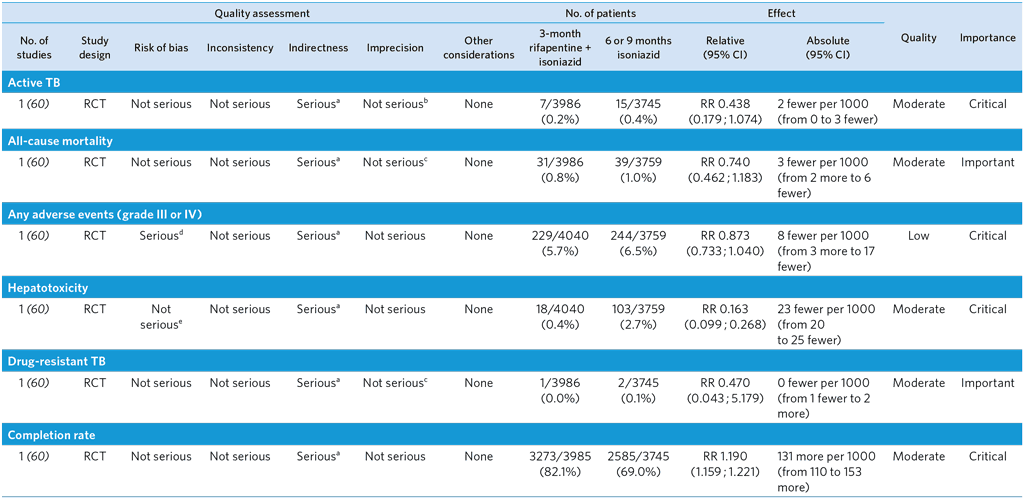
a No comparison with 6 months of isoniazid. The study included 2.7% HIV-positive participants. Although the trial was conducted in low TB incidence countries, this is unlikely to affect relative effect of rifapentine + isoniazid compared with isoniazid monotherapy. Downgraded by one level.
b Although the 95% CI of RR is wide, the number of events was small and the CI of absolute effect is narrow. The result also met pre-stated non-inferiority margin. Not downgraded.
c Although the 95% CI of RR is wide, the number of events was small and the CI of absolute effect is narrow. Not downgraded.
d An open-label design of the trial may have introduced ascertainment bias.
e Although the trial was open-label, this is unlikely to affect detection of hepatotoxicity, which is usually done by objective measurement (i.e. blood tests). Not downgraded.
3-month weekly rifapentine plus isoniazid or daily isoniazid monotherapy for treatment of TB infection in children and adolescents
Population: Children and adolescents
Comparison: 6 or 9 months isoniazid
Overall quality: moderate
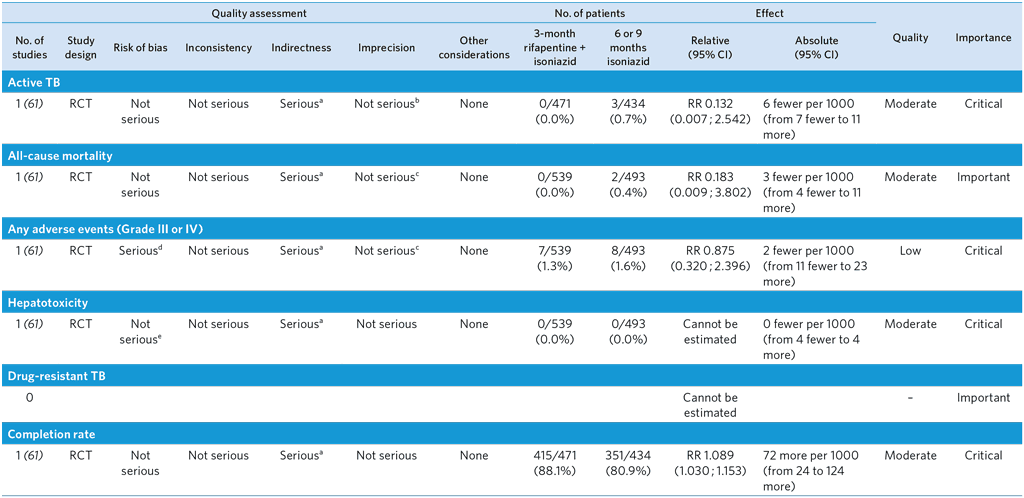
a No comparison against 6 months of isoniazid. Although the trial was conducted in low TB incidence countries, this is unlikely to affect relative effect of rifapentine + isoniazid compared with isoniazid monotherapy. Downgraded by one level.
b Although the 95% CI of the RR is wide, the number of events was small and the CI of absolute effect is narrow. The result also met pre-stated non-inferiority margin. Not downgraded.
c Although the 95% CI of the RR is wide, the number of events was small and the CI of absolute effect is narrow. Not downgraded.
d An open-label design of the trial may have introduced ascertainment bias.
e Although the trial was open-label, this is unlikely to affect detection of hepatotoxicity, which is usually done by objective measurement (i.e. blood tests). Not downgraded.
PICO 9: In pregnant and postpartum women, is isoniazid preventive treatment for TB as safe as other preventive treatment regimens?
Population: Isoniazid Preventive Therapy (IPT) compared to no IPT or placebo in pregnant women with HIV.
Bibliography:a (see references 62–65)
Overall quality of evidence rating: low
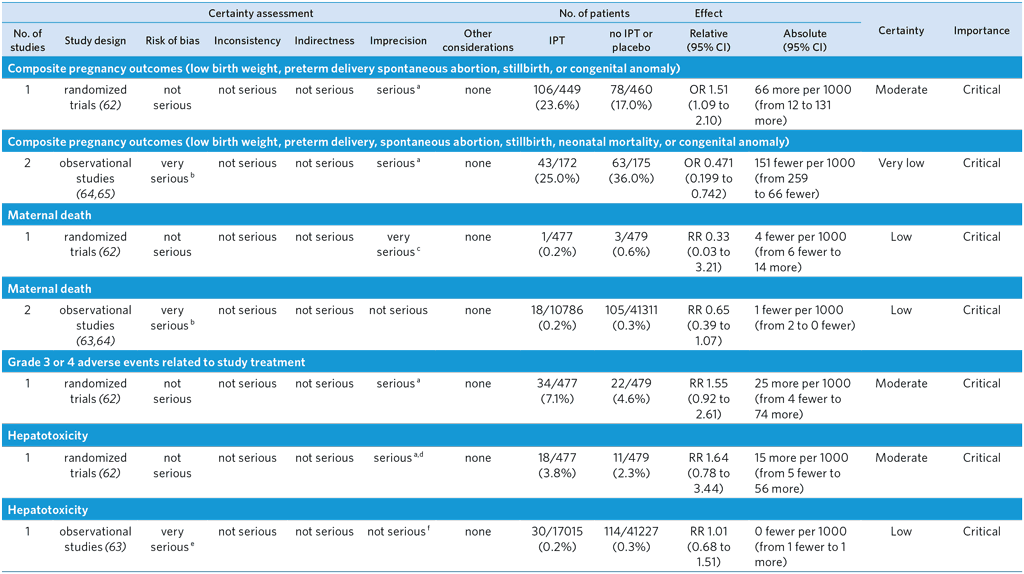
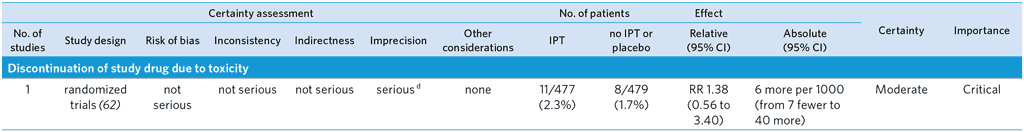
CI: Confidence interval; OR: Odds ratio; RR: Risk ratio
a Optimal information size not met.
b Bias due to confounding is considered serious. Important confounders are not fully accounted for.
c Large CI including both appreciable benefits and harms and very few events
d. CI includes both appreciable benefits and harms
e Confounding was not accounted for. Bias due to measurement of hepatotoxicity is considered serious since liver function tests were performed only if clinically indicated, which was likely to be influenced by knowledge of the receipt of IPT.
f Very large sample size and CI of absolute effect is very narrow.
Population: Immediate Isoniazid Preventive Therapy (IPT) compared to deferred IPT (12 weeks at post-partum) in pregnant women with HIV
Bibliography: (see reference 62)
Overall quality of evidence rating: moderate
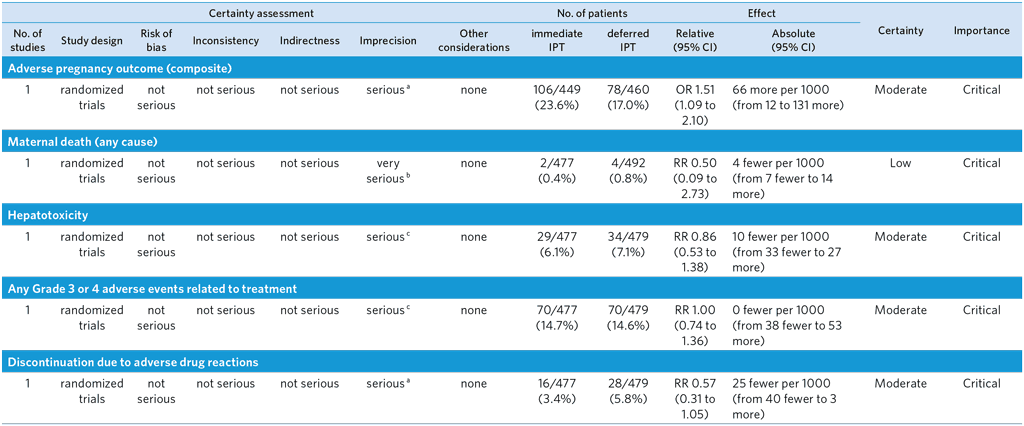
a Optimal information size not met.
b Large CI including both appreciable benefits and harms. Very few events.
c CI includes both appreciable benefit and harm.
PICO 10: Should 6 months of levofloxacin rather than other regimens or no TPT be recommended for people in contact with patients with MDR/RR-TB?
Author(s): Lawrence Mbuagbaw (McMaster University, Canada); Dennis Falzon (WHO Global Tuberculosis Programme, Switzerland); with contributions from Trinh Duong (University College London, United Kingdom); Dick Menzies (McGill University, Canada); Greg Fox (University of Sydney, Australia); Anneke Hesseling (Stellenbosch University, South Africa)
Question: 6 months of levofloxacin compared to other regimen or no TPT in people in contact with MDR/RR-TB
Setting: Two randomized controlled trials using 6 months of levofloxacin in contacts of MDR-TB in S Africa (TB CHAMP) and Viet Nam (VQUIN). We used results from a pooled analysis of individual study participant data to express estimates of effect, rather than the Bayesian analysis which to a large extent mirrored the results from the frequentist approach
Bibliography: (see references 66 and 67)
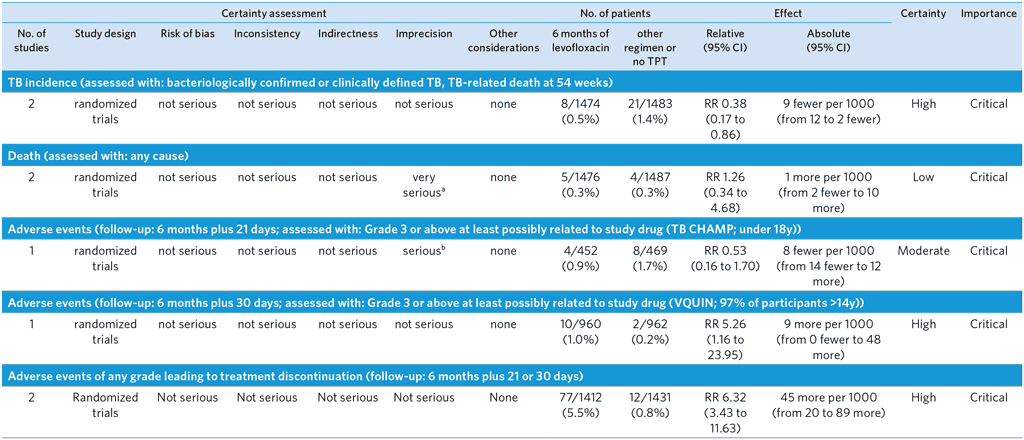
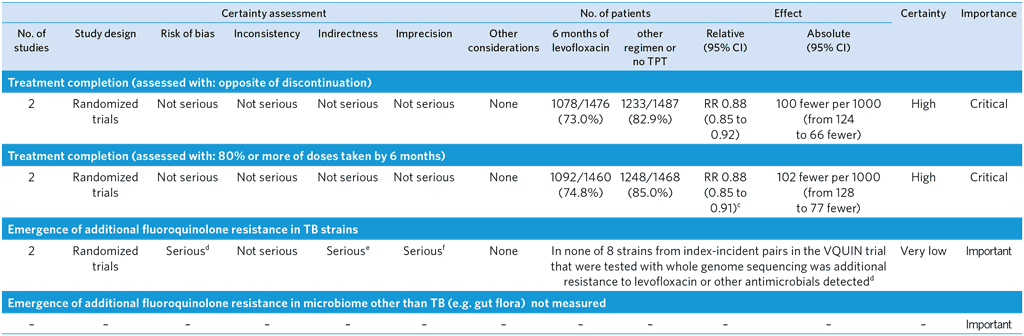
a We rated down two levels because the confidence intervals include appreciable harm and appreciable benefit: RR 1.26 (0.34 to 4.68)
b We rated down one level because the confidence intervals include appreciable harm and some benefit. RR 0.53 (0.16 to 1.70)
c Treatment completion in the levofloxacin arm was 86% in TB CHAMP (placebo arm: 86%) and 70% in VQUIN (placebo arm: 85%) – RRs 1.00 [95% CI 0.95 to 1.06] and 0.83 [0.79 to 0.87] respectively
d We rated down one level for risk of bias. The results are not from a randomized comparison. In VQUIN, of the 43 persons with suspected TB post-randomization, 17 had a laboratory-confirmed incident TB, in 4 of whom an isolate could not be recovered. Results were only available for 8/13. Of these 6 were in the placebo group and 2 from the LFX arm. In TB CHAMP, 14 individuals in the placebo arm and 7 in the LFX arm developed TB, of which 7 and 3 respectively with confirmed TB. No results for levofloxacin susceptibility were available for the strains isolated.
e We rated down one level for indirectness. Data was only available for VQUIN; all strains were from individuals aged over 15 years.
f We rated down one level for imprecision due to the small number of samples and zero events.
References
- Kasambira TS, Shah M, Adrian PV, Holshouser M, Madhi SA, Chaisson RE et al. QuantiFERON–TB Gold In-Tube for the detection of Mycobacterium tuberculosis infection in children with household tuberculosis contact. Int J Tuberc Lung Dis. 2011;15(5):628–34. doi:10.5588/ijtld.10.0555.
- Kenyon TA, Creek T, Laserson K, Makhoa M, Chimidza N, Mwasekaga M et al. Risk factors for transmission of Mycobacterium tuberculosis from HIV–infected tuberculosis patients, Botswana. Int J Tuberc Lung Dis. 2002;6(10):843–50. PMID:12365569.
- Klausner JD, Ryder RW, Baende E, Lelo U, Williame JC, Ngamboli K et al. Mycobacterium tuberculosis in household contacts of human immunodeficiency virus type 1– seropositive patients with active pulmonary tuberculosis in Kinshasa, Zaire. J Infect Dis. 1993;168(1):106–11. doi:10.1093/infdis/168.1.106.
- Bokhari SY, Ahmad A, Shaikh MY, Ahmad I. A study of tuberculosis contacts. J Pak Med Assoc. 1987;37(2):48–52. PMID:3106664.
- Biraro IA, Kimuda S, Egesa M, Cose S, Webb EL, Joloba M et al. The use of interferon gamma inducible protein 10 as a potential biomarker in the diagnosis of latent tuberculosis infection in Uganda. PLoS One. 2016;11(1):e0146098. doi:10.1371/journal.pone.0146098.
- Rutherford ME, Nataprawira M, Yulita I, Apriani L, Maharani W, van Crevel R et al. QuantiFERON(R)–TB Gold In–Tube assay vs. tuberculin skin test in Indonesian children living with a tuberculosis case. Int J Tuberc Lung Dis. 2012;16(4):496–502. doi:10.5588/ijtld.11.0491.
- Tornee S, Kaewkungwal J, Fungladda W, Silachamroon U, Akarasewi P, Sunakorn P. Risk factors for tuberculosis infection among household contacts in Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2004;35(2):375–83. PMID:15691140.
- Zelner JL, Murray MB, Becerra MC, Galea J, Lecca L, Calderon R et al. Bacillus Calmette–Guerin and isoniazid preventive therapy protect contacts of patients with tuberculosis. Am J Respir Crit Care Med. 2014;189(7):853–9. doi:10.1164/rccm.201310-1896OC.
- Tuberculosis Research Centre, Indian Council of Medical Research. Risk of tuberculosis among contacts of isoniazid–resistant and isoniazid–susceptible cases. Int J Tuberc Lung Dis. 2011;15(6):782–8. doi:10.5588/ijtld.09.0327.
- Radhakrishna S, Frieden TR, Subramani R, Santha T, Narayanan PR, Indian Council of Medical Research. Additional risk of developing TB for household members with a TB case at home at intake: a 15–year study. Int J Tuberc Lung Dis. 2007;11(3):282–8. PMID:17352093.
- Narain R, Nair SS, Rao GR, Chandrasekhar P. Distribution of tuberculous infection and disease among households in a rural community. Bull World Health Organ. 1966;34(4):639– 54. PMID:5296386.
- WHO Tuberculosis Chemotherapy Centre. An investigation of household contacts of open cases of pulmonary tuberculosis amongst the Kikuyu in Kiambu, Kenya. Bull World Health Organ. 1961;25(6):831–50. PMID:20604103.
- Andrews RH, Devadatta S, Fox W, Radhakrishna S, Ramakrishnan CV, Velu S. Prevalence of tuberculosis among close family contacts of tuberculous patients in South India, and influence of segregation of the patient on early attack rate. Bull World Health Organ. 1960;23:463–510. PMID:13683486.
- Loudon RG, Williamson J, Johnson JM. An analysis of 3,485 tuberculosis contacts in the city of Edinburgh during 1954–1955. Am Rev Tuberc. 1958;77(4):623–43. doi:10.1164/ artpd.1958.77.4.623.
- Triasih R, Robertson C, Duke T, Graham SM. Risk of infection and disease with Mycobacterium tuberculosis among children identified through prospective community-based contact screening in Indonesia. Trop Med Int Health. 2015;20(6):737–43. doi:10.1111/tmi.12484.
- Whalen CC, Zalwango S, Chiunda A, Malone L, Eisenach K, Joloba M et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PLoS One. 2011;6(2):e16137. doi:10.1371/journal.pone.0016137.
- Lewinsohn DA, Zalwango S, Stein CM, Mayanja–Kizza H, Okwera A, Boom WH et al. Whole blood interferon–gamma responses to Mycobacterium tuberculosis antigens in young household contacts of persons with tuberculosis in Uganda. PLoS One. 2008;3(10):e3407. doi:10.1371/journal.pone.0003407.
- Amanullah F, Ashfaq M, Khowaja S, Parekh A, Salahuddin N, Lotia–Farrukh I et al. High tuberculosis prevalence in children exposed at home to drug–resistant tuberculosis. Int J Tuberc Lung Dis. 2014;18(5):520–7. doi:10.5588/ijtld.13.0593.
- Ma N, Zalwango S, Malone LL, Nsereko M, Wampande EM, Thiel BA et al. Clinical and epidemiological characteristics of individuals resistant to M. tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. BMC Infect Dis. 2014;14:352. doi:10.1186/1471-2334-14-352.
- Rathi SK, Akhtar S, Rahbar MH, Azam SI. Prevalence and risk factors associated with tuberculin skin test positivity among household contacts of smear–positive pulmonary tuberculosis cases in Umerkot, Pakistan. Int J Tuberc Lung Dis. 2002;6(10):851–7. PMID:12365570.
- Lienhardt C, Fielding K, Sillah J, Tunkara A, Donkor S, Manneh K et al. Risk factors for tuberculosis infection in sub–Saharan Africa: a contact study in The Gambia. Am J Respir Crit Care Med. 2003;168(4):448–55. doi:10.1164/rccm.200212-1483OC.
- Jones–Lopez EC, White LF, Kirenga B, Mumbowa F, Ssebidandi M, Moine S et al. Cough aerosol cultures of Mycobacterium tuberculosis: Insights on TST / IGRA discordance and transmission dynamics. PLoS One. 2015;10(9):e0138358. doi:10.1371/journal.pone.0138358.
- Kifai EJ, Bakari M. Mantoux skin test reactivity among household contacts of HIV-infected and HIV un-infected patients with sputum smear positive TB in Dar es Salaam, Tanzania. East Afr J Public Health. 2009;6(2):211–8. doi:10.4314/eajph.v6i2.51786.
- Nunn P, Mungai M, Nyamwaya J, Gicheha C, Brindle RJ, Dunn DT et al. The effect of human immunodeficiency virus type–1 on the infectiousness of tuberculosis. Tuber Lung Dis. 1994;75(1):25–32. doi:10.1016/0962-8479(94)90098-1.
- Espinal MA, Perez EN, Baez J, Henriquez L, Fernandez K, Lopez M et al. Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet. 2000;355(9200):275–80. doi:10.1016/S0140-6736(99)04402-5.
- Hesseling AC, Mandalakas AM, Kirchner HL, Chegou NN, Marais BJ, Stanley K et al. Highly discordant T cell responses in individuals with recent exposure to household tuberculosis. Thorax. 2009;64(10):840–6. doi: 10.1136/thx.2007.085340.
- Guwatudde D, Nakakeeto M, Jones–Lopez EC, Maganda A, Chiunda A, Mugerwa RD et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158(9):887–98. doi:10.1093/aje/kwg227.
- Lees AW, Allan GW, Smith J, Tyrrell WF. Pulmonary tuberculosis in contacts: a ten year survey. Dis Chest. 1961;40:516–21. doi:10.1378/chest.40.5.516.
- Ahmad Khan F, Verkuijl S, Parrish A, Chikwava F, Ntumy R, El–Sadr W et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS. 2014;28(10):1463–72. doi:10.1097/QAD.0000000000000278.
- Nguyen DT, Bang ND, Hung NQ, Beasley RP, Hwang LY, Graviss EA. Yield of chest radiograph in tuberculosis screening for HIV–infected persons at a district–level HIV clinic. Int J Tuberc Lung Dis. 2016;20(2):211–7. doi:10.5588/ijtld.15.0705.
- den Boon S, White NW, van Lill SW, Borgdorff MW, Verver S, Lombard CJ et al. An evaluation of symptom and chest radiographic screening in tuberculosis prevalence surveys. Int J Tuberc Lung Dis. 2006;10(8):876–82. PMID:16898372.
- Ministry of Health. Report on National TB Prevalence Survey 2009–2010. Nay Pyi Taw: Department of Health; 2012 (https://www.myanmarhscc.org/wp-content/uploads/2019/09/prevelence_report.pdf).
- van’t Hoog AH, Meme HK, Laserson KF, Agaya JA, Muchiri BG, Githui WA et al. Screening strategies for tuberculosis prevalence surveys: the value of chest radiography and symptoms. PLoS One. 2012;7(7):e38691. doi:10.1371/journal.pone.0038691.
- Kapata N, Chanda-Kapata P, Ngosa W, Metitiri M, Klinkenberg E, Kalisvaart N et al. The prevalence of tuberculosis in Zambia: results from the first national TB prevalence survey, 2013–2014. PLoS One. 2016;11(1):e0146392. doi:10.1371/journal.pone.0146392.
- Kebede AH, Alebachew Z, Tsegaye F, Lemma E, Abebe A, Agonafir M et al. The first population–based national tuberculosis prevalence survey in Ethiopia, 2010–2011. Int J Tuberc Lung Dis. 2014;18(6):635–9. doi:10.5588/ijtld.13.0417.
- Senkoro M, Mfinanga S, Egwaga S, Mtandu R, Kamara DV, Basra D et al. Prevalence of pulmonary tuberculosis in adult population of Tanzania: a national survey, 2012. Int J Tuberc Lung Dis. 2016;20(8):1014–21. doi:10.5588/ijtld.15.0340.
- Law I, Sylavanh P, Bounmala S, Nzabintwali F, Paboriboune P, Iem V et al. The first national tuberculosis prevalence survey of Lao PDR (2010–2011). Trop Med Int Health. 2015;20(9):1146–54. doi:10.1111/tmi.12536.
- Adetifa IM, Kendall L, Bashorun A, Linda C, Omoleke S, Jeffries D et al. A tuberculosis nationwide prevalence survey in Gambia, 2012. Bull World Health Organ. 2016;94(6):433– 41. doi:10.2471/BLT.14.151670.
- Ayles H, Schaap A, Nota A, Sismanidis C, Tembwe R, De Haas P et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS One. 2009;4(5):e5602. doi:10.1371/journal.pone.0005602.
- Corbett EL, Zezai A, Cheung YB, Bandason T, Dauya E, Munyati SS et al. Provider–initiated symptom screening for tuberculosis in Zimbabwe: diagnostic value and the effect of HIV status. Bull World Health Organ. 2010;88(1):13–21. doi:10.2471/BLT.08.055467.
- Datta M, Radhamani MP, Sadacharam K, Selvaraj R, Rao DL, Rao RS et al. Survey for tuberculosis in a tribal population in North Arcot District. Int J Tuberc Lung Dis. 2001;5(3):240– 9. PMID:11326823.
- Gopi PG, Subramani R, Radhakrishna S, Kolappan C, Sadacharam K, Devi TS et al. A baseline survey of the prevalence of tuberculosis in a community in South India at the commencement of a DOTS programme. Int J Tuberc Lung Dis. 2003;7(12):1154–62. PMID:14677890.
- Ministry of Health. Report National TB Prevalence Survey, 2002 Cambodia. Phnom Penh: National Tuberculosis Control Programme, 2005 (https://niph.org.kh/niph/uploads/library/pdf/OT019_National_TB_Prevalence_Survey_2002_Cambodia.pdf).
- Mathad JS, Bhosale R, Balasubramanian U, Kanade S, Mave V, Suryavanshi N et al. Quantitative IFN–gamma and IL–2 response associated with latent tuberculosis test discordance in HIV–infected pregnant women. Am J Respir Crit Care Med. 2016;193(12):1421–8.
- Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomized double–blind, placebo– controlled trial. Lancet. 2014;384(9944):682–90. doi:10.1164/rccm.201508-1595OC.
- Mahomed H, Hawkridge T, Verver S, Abrahams D, Geiter L, Hatherill M et al. The tuberculin skin test versus QuantiFERON TB Gold(R) in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One. 2011;6(3):e17984. doi:10.1371/journal.pone.0017984.
- McCarthy KM, Scott LE, Gous N, Tellie M, Venter WD, Stevens WS et al. High incidence of latent tuberculous infection among South African health workers: an urgent call for action. Int J Tuberc Lung Dis. 2015;19(6):647–53. doi:10.5588/ijtld.14.0759.
- Sharma SK, Vashishtha R, Chauhan LS, Sreenivas V, Seth D. Comparison of TST and IGRA in diagnosis of latent tuberculosis infection in a high TB-burden setting. PLoS One. 2017;12(1):e0169539. doi:10.1371/journal.pone.0169539.
- Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F et al. The effectiveness of a 9–month regimen of isoniazid alone versus 3– and 4–month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11–year randomized study. Clin Infect Dis. 2007;45(6):715–22. doi:10.1086/520983.
- Galli L, Lancella L, Tersigni C, Venturini E, Chiappini E, Bergamini BM et al. Pediatric tuberculosis in Italian children: epidemiological and clinical data from the Italian Register of Pediatric Tuberculosis. Int J Mol Sci. 2016;17(6). doi:10.3390/ijms17060960.
- van Zyl S, Marais BJ, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Adherence to anti–tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis. 2006;10(1):13– 8. PMID:16466031.
- Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. New Engl J Med. 2018;379(5):440–53. doi:10.1056/NEJMoa1714283.
- Diallo T, Adjobimey M, Ruslami R, Trajman A, Sow O, Obeng Baah, J et al. Safety and side effects of rifampin versus isoniazid in children. N Engl J Med. 2018;379:454–63. doi:10.1056/NEJMoa1714284.
- Menzies D, Long R, Trajman A, Dion MJ, Yang J, Al Jahdali H et al. Adverse events with 4 months of rifampin therapy or 9 mnths of isoniazid therapy for latent tuberculosis infection: a randomized trial. Ann Intern Med. 2008;149(10):689–97. doi:10.7326/0003-4819-149-10-200811180-00003.
- Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am J Respir Crit Care Med. 2004;170(4):445–49. doi:10.1164/rccm.200404-478OC.
- Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873–90. doi:10.1002/ (sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i.
- Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz J et al. One month of rifapentine plusisoniazid to prevent HIV-related tuberculosis, N Engl J Med. 2019;380(11):1001– 11. doi:10.1056/NEJMoa1806808.
- Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11– 20. doi:10.1056/NEJMoa1005136.
- Sterling TR, Scott NA, Miro JM, Calvet G, La Rosa A, Infante R et al. Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS. 2016;30(10):1607–15. doi:10.1097/QAD.0000000000001098.
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven–Sizemore E et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–66. doi:10.1056/NEJMoa1104875.
- Villarino ME, Scott NA, Weis SE, Weiner M, Conde MB, Jones B et al. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015;169(3):247–55. doi:10.1001/jamapediatrics.2014.3158.
- Gupta A, Montepiedra G, Aaron L, Theron G, McCarthy K, Bradford S et al. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med. 2019;381(14):1333–46. doi:10.1056/NEJMoa1813060.
- Kalk EK, Heekes A, Mehta U, de Waal R, Jacob N, Cohen K et al. Programmatic review of safety and effectiveness of isoniazid preventive therapy in HIV-infected pregnant women on ART in routine care. Reprod Toxicol. 2018;80:155. doi:10.1093/cid/ciz1224.
- Salazar-Austin N, Cohn S, Lala S, Waja Z, Dooley KE, Hoffmann CJ et al. Isoniazid preventive therapy and pregnancy outcomes in HIV-infected women in the Tshepiso cohort. Clin Infect Dis. 2019;ciz1024. doi:10.1093/cid/ciz1024.
- Taylor AW, Mosimaneotsile B, Mathebula U, Mathoma A, Moathlodi R, Theebetsile I et al. Pregnancy outcomes in HIV-infected women receiving long-term isoniazid prophylaxis for tuberculosis and antiretroviral therapy. Infect Dis Obstet Gynecol. 2013;2013:1–5. doi:10.1155/2013/195637.
- Seddon JA, Garcia-Prats AJ, Purchase SE, Osman M, Demers Am Hoddinott G et al. Levofloxacin versus placebo for the prevention of tuberculosis disease in child contacts of multidrug resistant tuberculosis: study protocol for a phase III cluster randomized controlled trial (TB-CHAMP). Trials. 2018;19(1):693. doi:10.1186/s13063-018-3070-0.
- Fox GJ, Nguyen CB, Nguyen TA, Thuy Tran P, Marais BJ, Graham SM et al. Levofloxacin versus placebo for the treatment of latent tuberculosis among contacts of patients with multidrug-resistant tuberculosis (VQUIN MDR trial): a protocol for a randomized controlled trial. BMJ Open. 2020;10(1):e033945. doi:10.1136/bmjopen-2019-033945.
 Retour
Retour