Liens transversaux de livre pour 4. Respiratory protection
Respiratory protection should be implemented as a part of a package of TB IPC interventions. However, inappropriate implementation of respiratory protection measures, or reliance on these measures alone, may create a false sense of security and increase the risk of TB (13). The available studies assessing the effectiveness of respiratory protection in reducing the risk of M. tuberculosis transmission are highly heterogeneous. The absolute reduction in TST conversion ranged from 4.3% to 14.8% (13). However, since the studies usually test a package of TB IPC interventions, direct evidence on the role of respiratory protection is lacking (13). The WHO GDG noted that the overall protection depends on whether respirators have been properly fit-tested and maintained, and on the quality of training and the way the respirators are used.
WHO recommends using particulate respirators, within the framework of a respiratory protection programme, to reduce M. tuberculosis transmission to health workers, individuals attending health care facilities or other people in settings with a high risk of transmission. The recommendation that health care workers should use particulate respirators applies to all health care and nonhealth congregate settings (e.g. correctional facilities), and other settings where health services are provided to individuals with presumed and confirmed TB (e.g. refugee and asylum centres). The introduction of particulate respirators for health care workers should be part of a more comprehensive respiratory protection programme that is in line with occupational safety and health measures. Particulate respirators are effective only if people know how to use them. Respiratory protection should be offered to all personnel entering high-risk areas, particularly health care workers living with HIV or those who are exposed to people with infectious TB and DR-TB. Respirators may also be used by community workers or family members caring for patients with TB who are infectious.
A comprehensive respiratory protection component of a programme has the following elements:
- a TB IPC focal person at the health facility or congregate setting to coordinate the implementation of the respiratory protection programme;
- allocation of dedicated funding and human resources to ensure availability of commodities such as medical masks and respirators;
- funds for the development of education material and training of staff in appropriate application, use and disposal of respirators;
- a choice of appropriate respirators that meet global standards for protection (e.g. N95, FFP2 or FFP3) in different settings; the procurement team should consider different sizes to fit the range of staff members in the facility;
- written SOPs that are made available to all users and displayed at strategic locations;
- a plan for respirator fit-testing for all users, including fit-testing after every change of brand or make of the respirators; as a minimum, all health care workers must do a seal check before wearing a respirator, as shown in Fig. 4.1 (see Sect 4.1.1 for more details on conducting seal and fit test) (43), and systematic records of fit-testing must be maintained;
- mechanisms to ensure that respirators are used by all personnel entering high-risk areas, health care workers living with HIV and care providers for infectious TB patients; and
- general health screening of those using respirators, to assess whether they can perform duties for long hours wearing a respirator – those with impaired lung function (e.g. asthma or chronic obstructive pulmonary disease) may be unable to perform duties with respirators and should be assigned to different tasks.
4.1 Particulate respirators
TB was the first disease proven to be transmitted by the airborne route. More recently, viable M. tuberculosis have been isolated from cough and exhaled aerosols from persons with TB. Most of the particles in these aerosols are small and can be inhaled and deposited deep in lung tissues, where they can establish infection and progress to disease among those who are vulnerable. Respirators reduce the risk of inhaling such infectious particles and can help to mitigate the occupational risk of TB among health care workers. Although not all individuals with TB are infectious, there is currently no accurate way to determine which people are most infectious. Further, the range of infectious aerosol production from individuals with TB varies more than 1000-fold, which may explain why some people are “super-spreaders” whereas others are not infectious (44). From an TB IPC stand point, the most infectious individuals with TB are those who are not yet on appropriate TB treatment (20).
The use of respirators in health care settings is relatively new; they were first implemented in the early 1990s for the protection of health care workers managing MDR-TB outbreaks in the United States of America (USA). The respirators contain a filter with tiny pores that can block infectious droplets, thus protecting the user from inhaling them when the respirator is fitted closely to the face. Standards used for respirators differ between the USA and Europe. In the USA, N95 respirators are recommended, where the “N” refers to its approval for use against non-oil aerosols, and the ”95” means that the filter material is at least 95% efficient at removing the particles of the most penetrating size (0.3 μm). The equivalent respirators in the European standard are FFP2 and FFP3. FFP2 filters out more than 94% of the particles (0.4 μm) and FFP3 filters out more than 98%. This filtration capability, however, does not directly translate into, for example, 95% protection with N95. The protection depends on the respirator being worn properly and fitting well. Although N95 or FFP2 are adequate for protection for general use for TB IPC, a higher grade of protection (e.g. FFP3) may be needed in specific settings (e.g. when undertaking aerosol-generating procedures on infectious TB patients).²⁰
Fig. 4.1. Examples of particulate respirators²¹
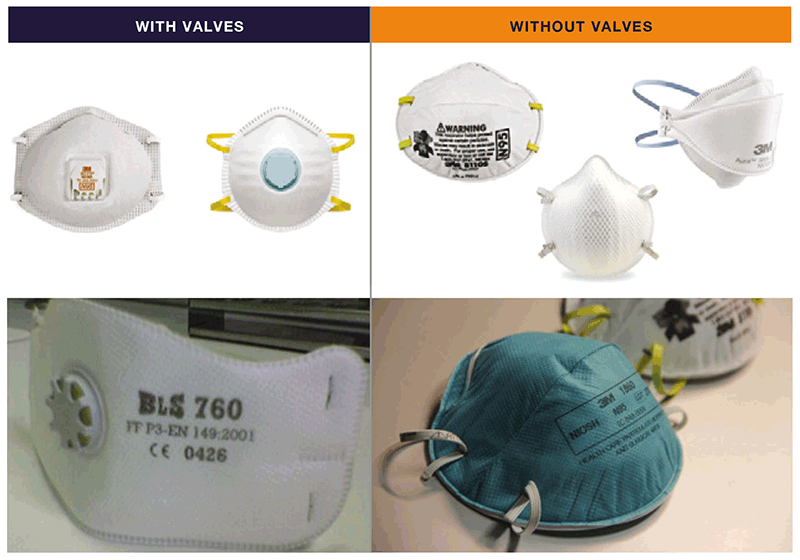
Source: Curry International Tuberculosis Center (2022) (20) (top two images; images supplied by Pexels/CDC and GB Migliori (bottom two images).
Fig. 4.2. Use of respirators by health care workers
Source: Aurum Institute, South Africa.
Respirators have a substantial cost, considering the volumes that may be required for programmatic implementation. Disposable respirators do not need to be discarded at the end of each task; however, they should be discarded when they are no longer in their original working condition because of contamination, structural defects or wear.22 They can be reused for 6–10 hours by the same person (or as indicated by manufacturer). The respirators should not be touched while being worn, hand hygiene should be performed when a used mask is touched or disposed of, and a respirator should be stored in a paper or tissue container (not plastic) to allow it to dry between each use. Users should not try to clean or decontaminate a disposable respirator (except in the case of elastomeric respirators (not depicted in Fig.4.2), which can be cleaned and disinfected after removing filters). It is important to dispose of the respirators correctly, following the national requirement for contaminated medical waste disposal.
4.1.1 Respirator fit-testing
The major limitation in the protection offered by respirators is the potential for excessive air leakage between the respirator and the face. Fit-testing is required to assure a tight fit of the respirator assigned to the user. A key complaint of users is that respirators are less comfortable than medical masks because of the increased effort required to inhale air through the filter material. Hence, if a particulate respirator feels as comfortable as a medical mask, it may not be providing adequate protection. Fit-testing of respirators is essential when they are newly introduced in the facility, or when a new batch of respirators is received. Fit-testing provides a way to determine which respirator model and size fits the wearer best; it also provides an opportunity to assess whether the wearer can use the respirator properly. Periodic fit-testing can thus serve as an effective, hands-on training opportunity, including on other IPC interventions included in the employee training and retraining curriculum. Fit-testing should be offered to all new employees and repeated annually for all health care workers. The frequency of fit-testing may also be determined by:
- the risk of transmission of M. tuberculosis (e.g. high TB prevalence or high-risk settings);
- changes in facial features of the wearer or medical conditions that would affect respiratory function;
- changes in the physical characteristics of the respirator (size, type, model or make); and
- national policy on frequency of fit-testing.
Fit-testing²³may be carried out using a test agent for qualitative detection, assessed by the wearer’s sense of taste or smell, or involuntary cough (irritant smoke); it may also be done using quantitative measurement with an instrument (i.e. ratio of the aerosol concentration outside the respirator to the aerosol concentration inside) to verify the respirator’s fit.²⁴ Fig. 4.3 provides an example of a qualitative respirator fit-testing kit that typically includes a hood with collar, bottles with sensitivity and challenge test solutions, and two nebulizers for injection of the test solutions.
Fig. 4.3. Example of a qualitative respirator fit-testing kit
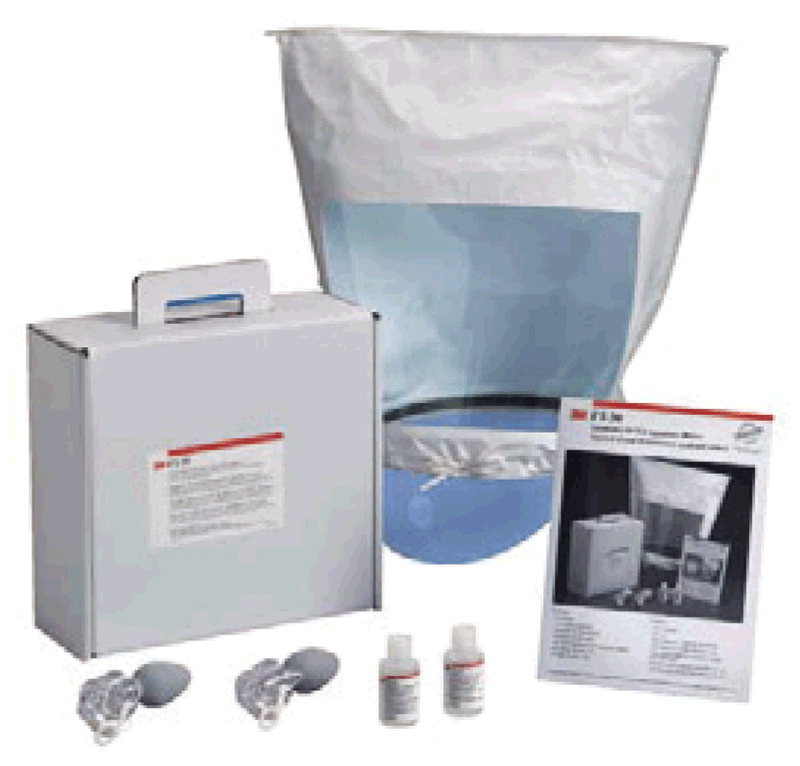
Source: Stop TB Partnership (2009) (12).
Test solutions for fit-testing
There are four methods for testing, using four different types of test solution: isoamyl acetate, irritant aerosol, saccharin and BitrexTM (denatonium benzoate). Tests using saccharin or BitrexTM are relatively easy to implement and can be used to test all types of disposable and elastomeric negative-pressure respirators (N95, 99 and 100 series, and FFP2 and FFP3). These tests use the subject’s sense of taste and involve the use of a small test hood, as shown in Fig. 4.4.
Example of fit-testing procedure
The subject is expected to avoid eating or drinking anything (except water) for 15 minutes before the test, to avoid anything that has been eaten or drunk being confused with the taste of the test solution. The fit-testing is completed in two steps – a sensitivity test followed by a challenge test – and results are systematically documented.
Fig. 4.4. Fit-testing using a test hood
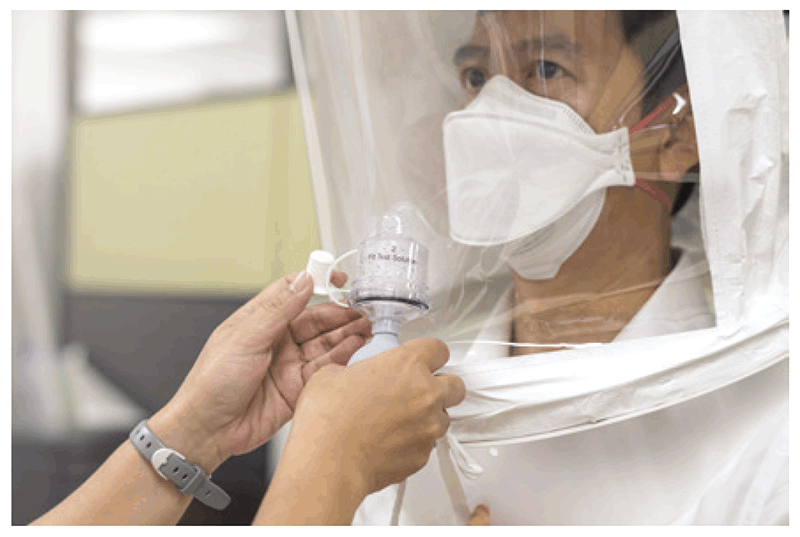
Source: Curry International Tuberculosis Center (2022) (20).
Sensitivity test
A sensitivity test is performed without the respirator. The tester injects 10 squeezes of the sensitivity test solution through a hole in the transparent visor of the hood. If the subject can taste the solution, the sensitivity test is complete. If the subject cannot taste the solution after 10 squeezes, an additional 10–20 squeezes are injected, until the subject detects the taste. The hood is then removed, and the subject is given a few minutes for the taste of the test solution to fade. This sensitivity test allows the subject to become familiar with the test solution. The number of squeezes needed for the subject to taste the solution during the sensitivity test is recorded (10, 20 or 30), and the same number of squeezes is used for the challenge test.
Challenge test
The aim of the challenge test is to understand whether a specific model of respirator used close to the face fits perfectly and prevents leakage around the edges. The subject puts the respirator on and is then asked to wear the hood. Several squeezes of the fit test solution (10, 20 or 30 – the same number as used in the sensitivity test) are injected under the hood. If the respirator is correctly placed and fits the subject’s face well, the previously recognized test solution cannot be detected, despite injection of the test solution. The subject is then asked to complete seven actions: breathe normally, breathe deeply, move the head from side to side, move the head up and down, talk nonstop, jog or walk on the spot, and return to breathing normally (10), to mimic the practical actions performed by health care workers during patient care. Each of these seven steps is continued for 1 minute. If the subject completes the series without detecting the taste of the solution, the test is passed (i.e. the respirator is an appropriate fit for the subject). If the taste is detected during any of the seven steps, the fit test has failed, meaning that the subject may not be protected by the respirator. In cases of test failure, the tester should wait for 15 minutes and then perform the test again. A second failure indicates the need for a different size or model of respirator. Fig. 4.5 depicts the points where air leakage frequently occurs (11).
Fig. 4.5. Points on a particulate respirator where air leakage is likely to occur
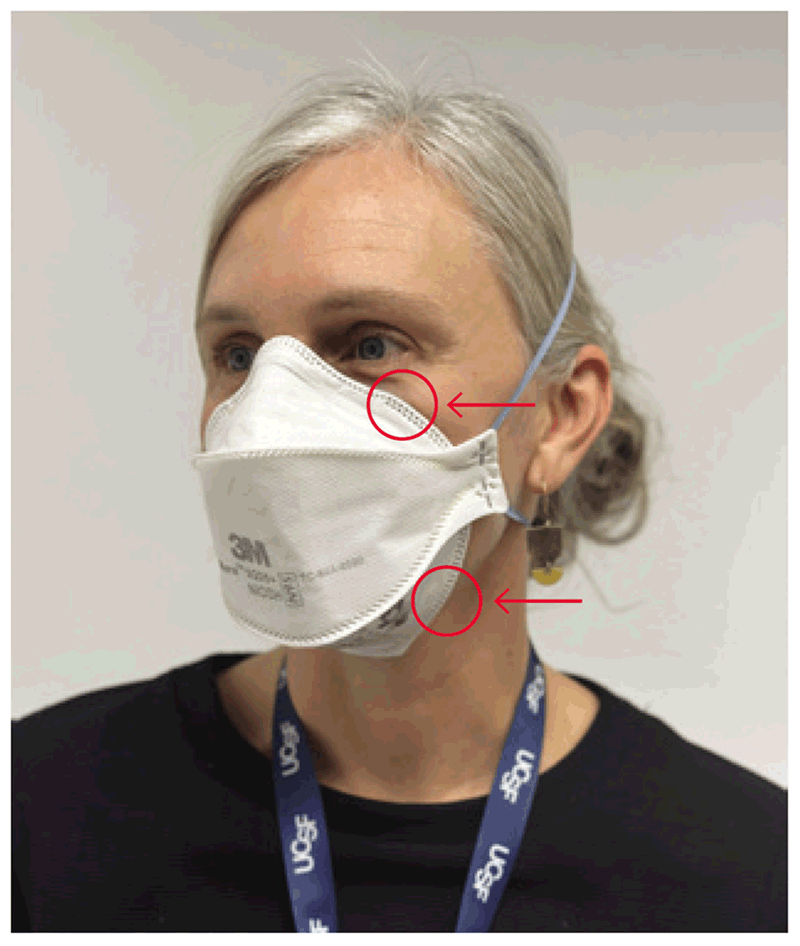
Source: Curry International Tuberculosis Center (2022) (20).
Seal check on a particulate respirator
It is important to implement the plan for respirator fit-testing for all users, including fit-testing after every change of brand or make of the respirators. As a minimum, all health care workers must do a seal check before wearing a respirator, as shown in Fig. 4.6 (43).
Fig. 4.6. How to perform a seal check on a particulate respirator
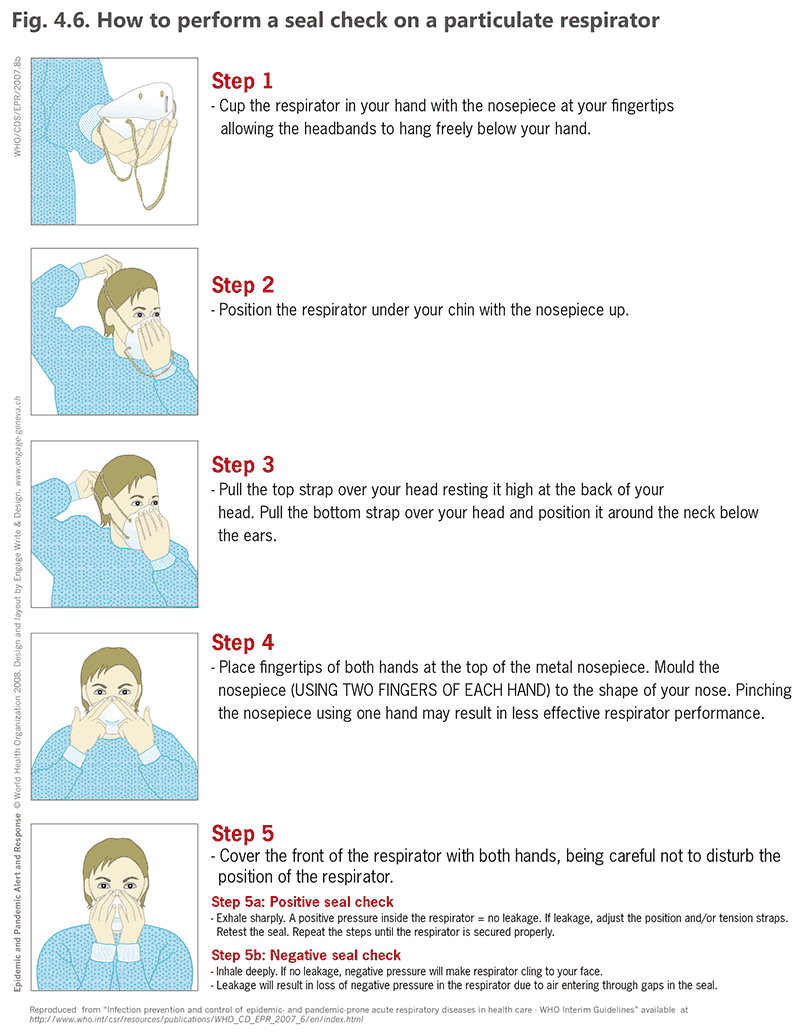
Source: WHO (2007) (43).
4.2 Medical masks
Medical masks are traditionally used for keeping the surgical field or environment sterile. They are also used in health care settings as personal protection from infection spread via the droplet route. However, medical masks offer minimal protection against airborne M. tuberculosis. They consist of gauze or tissue without any filter to block infectious droplets. Hence, they are best suited to simply reducing the release of infectious aerosols into the room air by individuals with infectious TB. The use of medical masks by individuals with TB has been shown to decrease transmission to guinea pigs by over 50% (46).
Fig. 4.7. Medical mask used to reduce the release of infectious aerosols
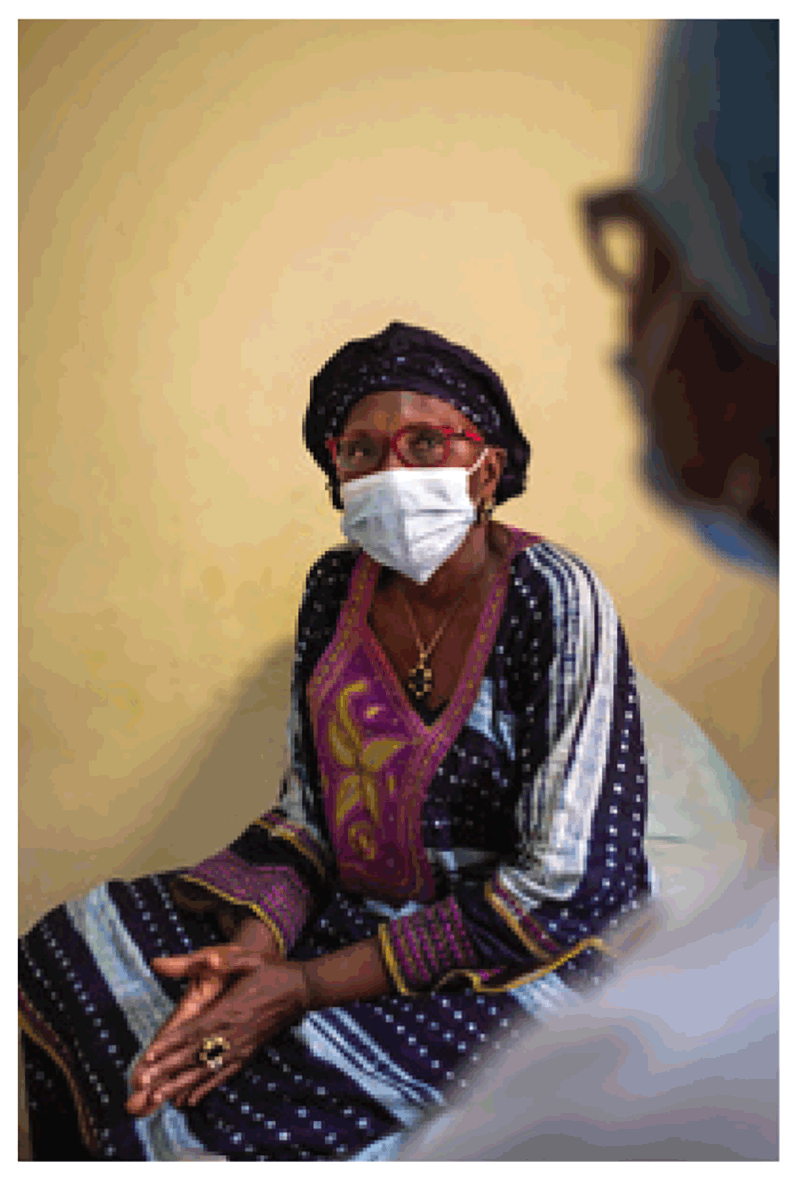
Source: WHO / Blink Media – Ricci Shryock.
If an individual is bacteriologically positive or not responding to TB treatment, the person should wear a medical mask when in contact with other people, particularly in areas that are poorly ventilated. The mask should have a nasal bar that can fit around the user’s nose, and it should completely cover their nose and mouth. The mask must be replaced at least once a day, or if it becomes wet or damaged. Given the increased work of breathing associated with pulmonary TB, it may not be appropriate to force an individual with TB to wear a respirator, but it may be reasonable to ask them to wear a medical mask. The individual may be able to remove the medical mask for large portions of the day (e.g. when they are alone, outside or sleeping); this is important because the mask restricts air movement and is often not comfortable.
Medical masks are part of the standard medical supplies procured by health services. Hence, the provision of medical masks to patients and the related education programme may incur minimal additional costs in health care and in congregate settings.
Source: WHO / Khalil Ashawi
20 Tracheal intubation, noninvasive ventilation (e.g. bilevel positive airway pressure and continuous positive airway pressure), tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, bronchoscopy, sputum induction by using nebulized hypertonic saline, dentistry and autopsy procedures.
21 Similar examples of respirators certified for use in different countries include KN95 in China, P2 in Australia and New Zealand, Korea 1st class (or KF94) in the Republic of Korea, DS2 in Japan and PFF2 in Brazil.
22 US Department of Labor, Occupational Safety and Health Administration (OSHA).
23 Additional information on fit-testing may be found in (40) and on the OSHA website (https://www.osha.gov/).
24 A sample video is available, describing both quantitative and qualitive fit-testing (45).
 Retour
Retour