Liens transversaux de livre pour 3.2 TB diagnosis
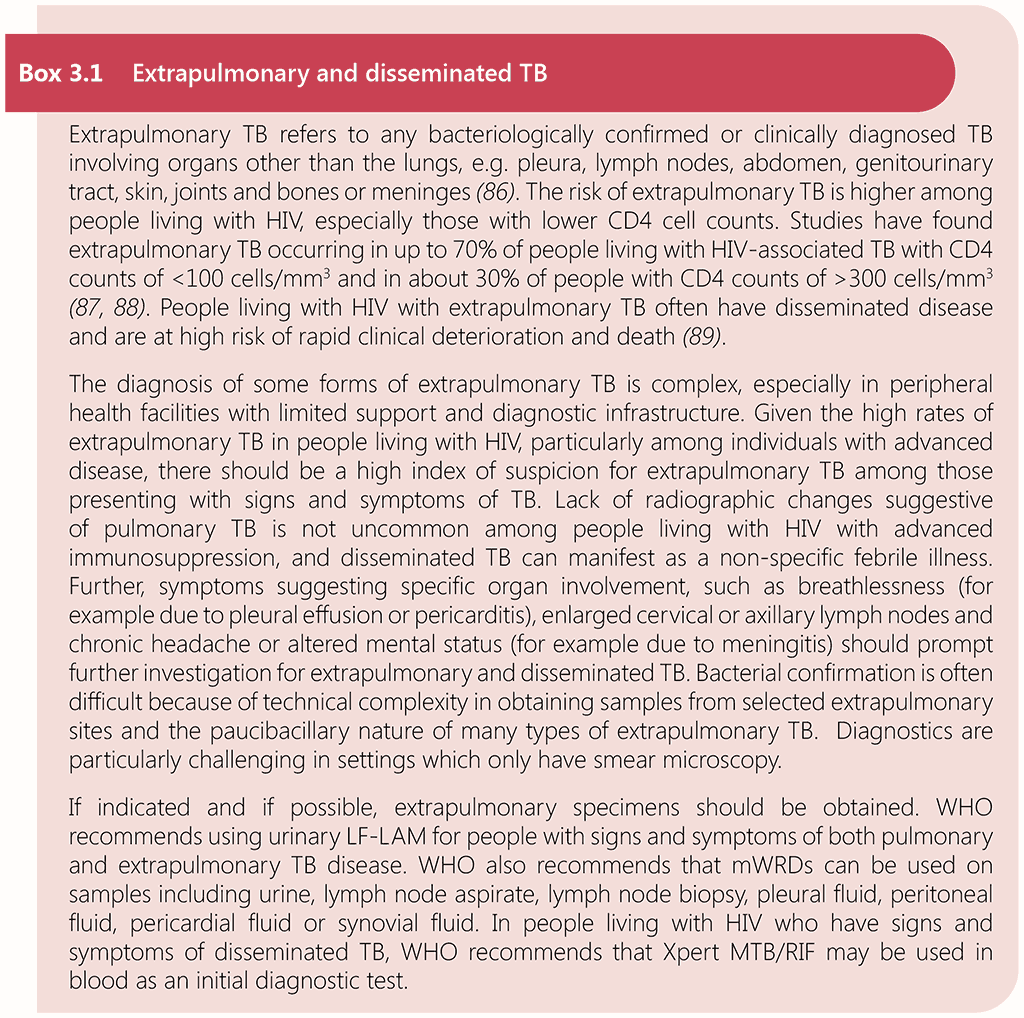
People living with HIV may have an atypical clinical picture, with higher rates of extrapulmonary and disseminated TB, especially those with advanced disease, see Box 3.1. Up to one third of people with HIV-associated TB are unable to produce sputum (83). HIV-associated TB is disseminated in up to 88% of post-mortem cases, likely due to its non-specific clinical presentation and a high proportion of bacteriologically negative and radiographically non-specific disease (2). Given the challenges in diagnosing TB in people living with HIV and the high risk of mortality, a diagnostic strategy that has high sensitivity is critical.
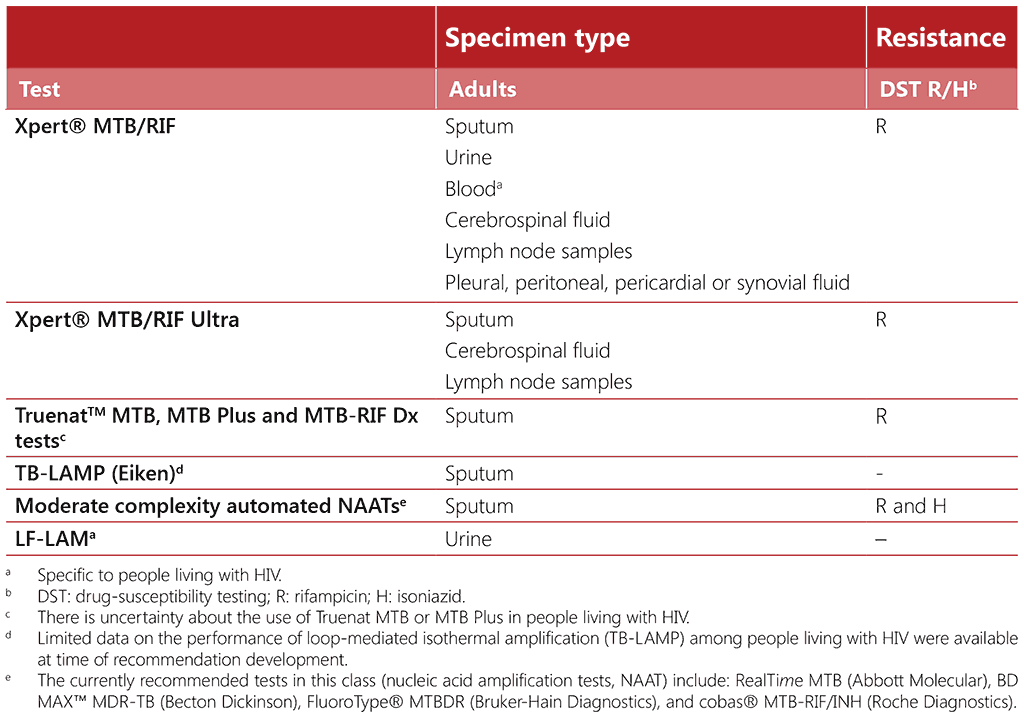
The TB diagnostic tests recommended by WHO (12) belong to two broad groups: (i) initial tests for diagnosing TB, with or without at least rifampicin resistance detection, and (ii) follow-on tests aimed at detecting additional drug resistance once a TB diagnosis is made. Table 3.1 describes initial tests for diagnosing TB which are applicable to everyone, with LF-LAM and the use of mWRDs in blood being specific to people living with HIV. Follow-on tests are not included in this chapter but covered in the relevant TB guidelines (12) and operational handbook (84). Further details on each of these tests can be found in WHO consolidated guidelines on tuberculosis. Module 3: diagnosis. Rapid diagnostic tests for TB detection (12).
In many high TB burden settings, sputum-smear microscopy remains the primary diagnostic technique for evaluating individuals presenting with signs and symptoms of TB. However, sputumsmear microscopy has a low sensitivity, particularly in people living with HIV and among people who have difficulty in producing sputum or have paucibacillary sputum. Furthermore, sputumsmear microscopy cannot distinguish drug-susceptible strains from drug-resistant strains. WHO recommends that TB programmes transition to replacing microscopy as the initial diagnostic test with mWRDs that detect Mycobacterium tuberculosis complex (MTBC). The initial diagnostic test for TB among people living with HIV should therefore be an mWRD for testing sputum or another sample, as indicated (84). In addition, a urine LF-LAM test can be used in parallel with an mWRD to assist in the diagnosis of TB disease in eligible people living with HIV (84), alongside other clinical, radiological or laboratory procedures for detecting pulmonary and extrapulmonary TB.
Table 3.1. WHO-recommended rapid diagnostic tests as initial tests for TB diagnosis
3.2.1 Initial diagnostic tests for diagnosis of TB with drug-resistance detection
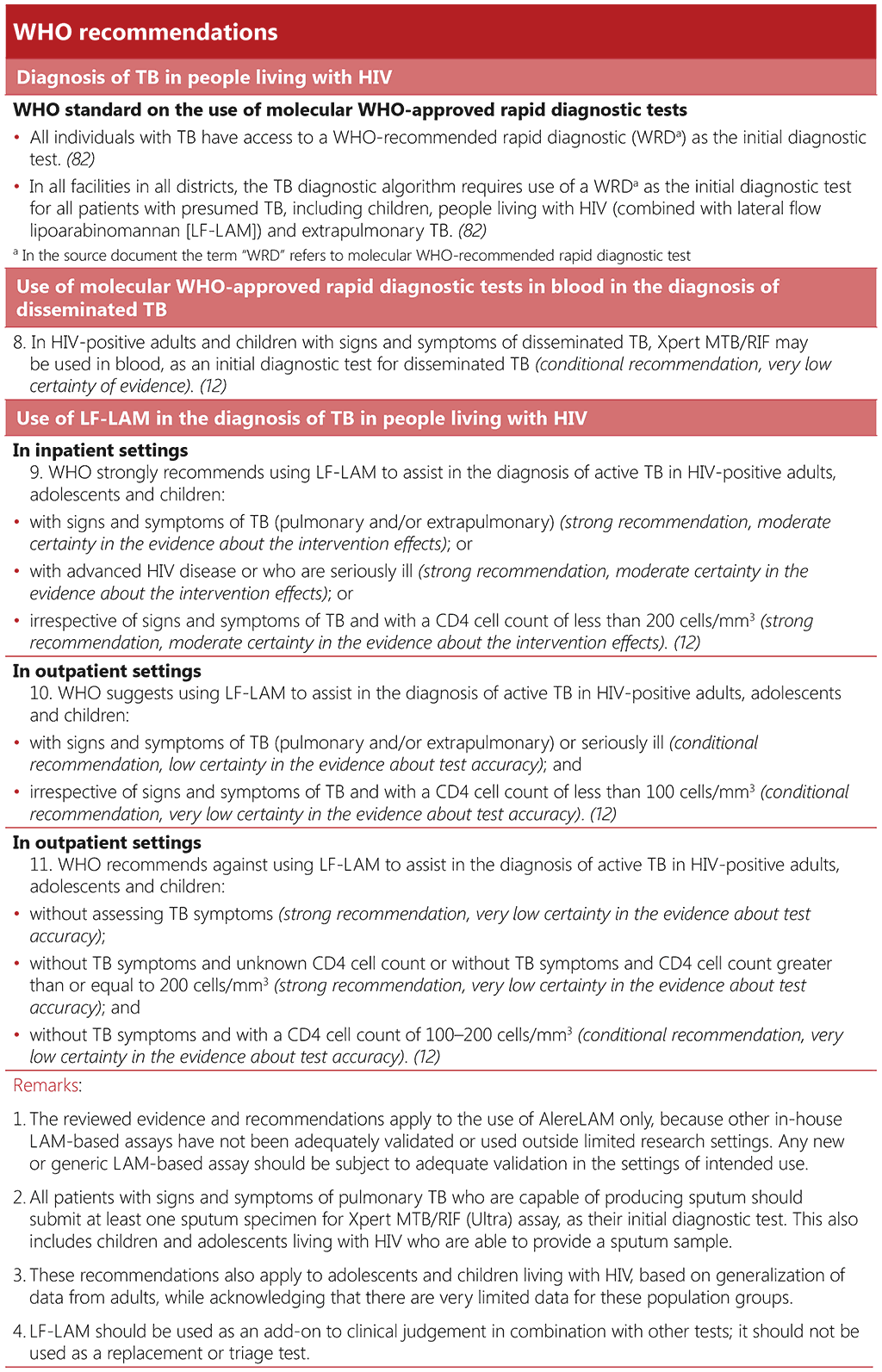
The WHO standard: universal access to rapid tuberculosis diagnostics includes two benchmarks that require that all individuals have access to an mWRD as an initial diagnostic test and that all facilities use an algorithm that includes an mWRD as an initial diagnostic test, alongside urinary lateral flow lipoarabinomannan, for people living with HIV for both pulmonary and extrapulmonary TB (82).
In 2011, WHO first recommended Xpert MTB/RIF as a replacement for smear microscopy for people being assessed for multidrug-resistant TB (MDR-TB) or HIV-associated TB. Since 2020, WHO has recommended mWRDs for the initial diagnosis of TB, instead of smear microscopy, for all people being evaluated for TB disease, regardless of HIV status (85). Molecular WHO-recommended rapid diagnostic tests are also prescribed for diagnosis of most forms of extrapulmonary TB. In addition, for people living with HIV who have signs and symptoms of disseminated TB, WHO recommends that Xpert MTB/RIF may be used in blood, as an initial diagnostic test for disseminated TB. It is still critical that people living with HIV have access to mWRDs as early as possible in the diagnostic cascade.
Programmes should ensure that when TB samples cannot be tested at the same location where they are collected, the TB sample transportation network has good links with health facilities providing HIV care, to ensure fast turnaround from presentation to initiation of TB treatment.
The use of multi-disease diagnostic platforms presents an opportunity for increasing access to mWRDs. Several of the molecular diagnostic platforms for diagnosing TB are also widely used in early infant diagnosis of HIV and viral load monitoring for people living with HIV, as well as, for example, for diagnosis of SARS-CoV-2, viral hepatitis, and for antimicrobial resistance detection of bacterial pathogens. Countries may also consider integrating sample transport systems for TB and HIV. Multi-disease testing and integrated sample transportation can have the advantage of shared financial costs and functionality, and improved efficiencies. Turnaround time from initial presentation with presumptive TB to treatment initiation should not, however, be compromised.
Multi-disease testing is mainly useful where the number of tests by individual programmes is small, relative to the testing capacity within a particular setting. In contrast, scenarios where there are large TB and HIV testing needs, and infrastructure is installed to meet the demand to match the requirements of each disease, multi-disease testing may be less relevant. Nonetheless, the burden of disease and testing volumes change over time; hence, the use of equipment should be monitored, and programmes may need to adapt.
3.2.2 Urine LF-LAM
Urine LF-LAM is an immunocapture assay based on the detection of the mycobacterial LAM antigen in urine, which can be used as a point-of-care test for people living with HIV being evaluated for TB. Although the assay has low sensitivity, it can be used as a fast (test time <15 min) rule-in test for TB among people living with HIV, especially where a rapid TB diagnosis is critical for survival. Bedside LF-LAM guided initiation of TB treatment in hospital inpatients with HIV, with presumptive TB, was associated with reduced 8-week mortality (90). Including LF-LAM in TB diagnostic algorithms for people living with HIV who are severely ill has been shown to be cost-effective (90). The Alere/Abbot Determine TB LAM Ag is currently the only commercially available urine LF-LAM test endorsed by WHO, since this was the only LAM-based assay that had been adequately validated or used outside limited research settings at the time of updating the WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection, 2021 update (84). This test can be used in any setting, unlike other diagnostics which have specific infrastructure requirements.
Due to its low sensitivity, LF-LAM should not be used as a screening test, and a negative LF-LAM test result does not rule out TB. Furthermore, the detection of mycobacterial LAM antigen in the urine does not provide any information on drug resistance, and people living with HIV are particularly at risk of dying from multidrug-resistant TB (91, 92). Therefore, testing with an mWRD is critical for ensuring TB is not missed and the correct treatment is provided. For their initial diagnostic test, all people with signs and symptoms of pulmonary TB who are able to produce sputum should have at least one sputum specimen submitted for an mWRD assay. For people who are unable to produce sputum, alternative specimens should be submitted for an mWRD, as appropriate. LF-LAM results (test time <15 min) are likely to be available before mWRD results; hence, treatment decisions should be based on the LF-LAM result while awaiting the results of other diagnostic tests. Box 3.2 below outlines key steps to consider for the introduction of all new diagnostic tests, including LF-LAM. For further details, see the WHO operational handbook on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection, 2021 update (84).

3.2.3 Clinical diagnosis of TB
The decision to carry out treatment based on a clinical diagnosis of TB, also referred to as presumptive TB treatment or empirical treatment, is of relevance among seriously ill people living with HIV who are at high risk of mortality and among whom establishing a TB diagnosis may be difficult. The rationale for empirical treatment is to prevent the death of people living with HIV in situations when expedited diagnosis of TB is not possible or feasible because of the person’s clinical condition, and there is limited access to TB diagnostic services (84). WHO algorithms include initiating TB treatment for people living with HIV based on the judgement of the clinician (84, 89). If results from LF-LAM and mWRD are negative, or if an mWRD is unavailable, further assessments and investigations may include the assessment of risk factors such as malnourishment, poor living conditions, a clinical symptoms assessment, chest X-ray, and referral for mWRD testing or culture. In line with WHO’s recommended package of care for advanced HIV disease, empirical treatment of pneumocystis or bacterial pneumonia should be considered for people with severe respiratory distress. If after 3–5 days of antibiotic treatment there is clinical worsening or no improvement and the person is seriously ill with danger signs, empirical TB treatment is indicated.
It should be emphasized that every effort should be made to confirm the diagnosis of TB after initiating empirical treatment and that treatment should be stopped only if bacteriological, histological or strong clinical evidence indicates an alternative diagnosis.
 Retour
Retour