Liens transversaux de livre pour 3.5 Prevention of TB
3.5.1 TB preventive treatment
It is estimated that a quarter of the world’s population has been infected with TB bacilli. TB infection, previously called latent TB infection (LTBI), is defined as a state of persistent immune response to stimulation by M. tuberculosis antigens with no evidence of clinically manifest TB disease (107, 108). People living with HIV have a higher risk of developing TB disease compared to the general population, even when on ART and with a high CD4 cell count (109, 110). Among people living with HIV, TB preventive treatment provides an added benefit to ART alone in reducing both TB incidence and overall mortality, regardless of CD4 count (111, 112). TB preventive treatment for people living with HIV should be a core component of the HIV package of care and should primarily be the responsibility of national HIV and AIDS programmes and HIV service providers (17).
3.5.1.1 Eligibility for TB preventive treatment
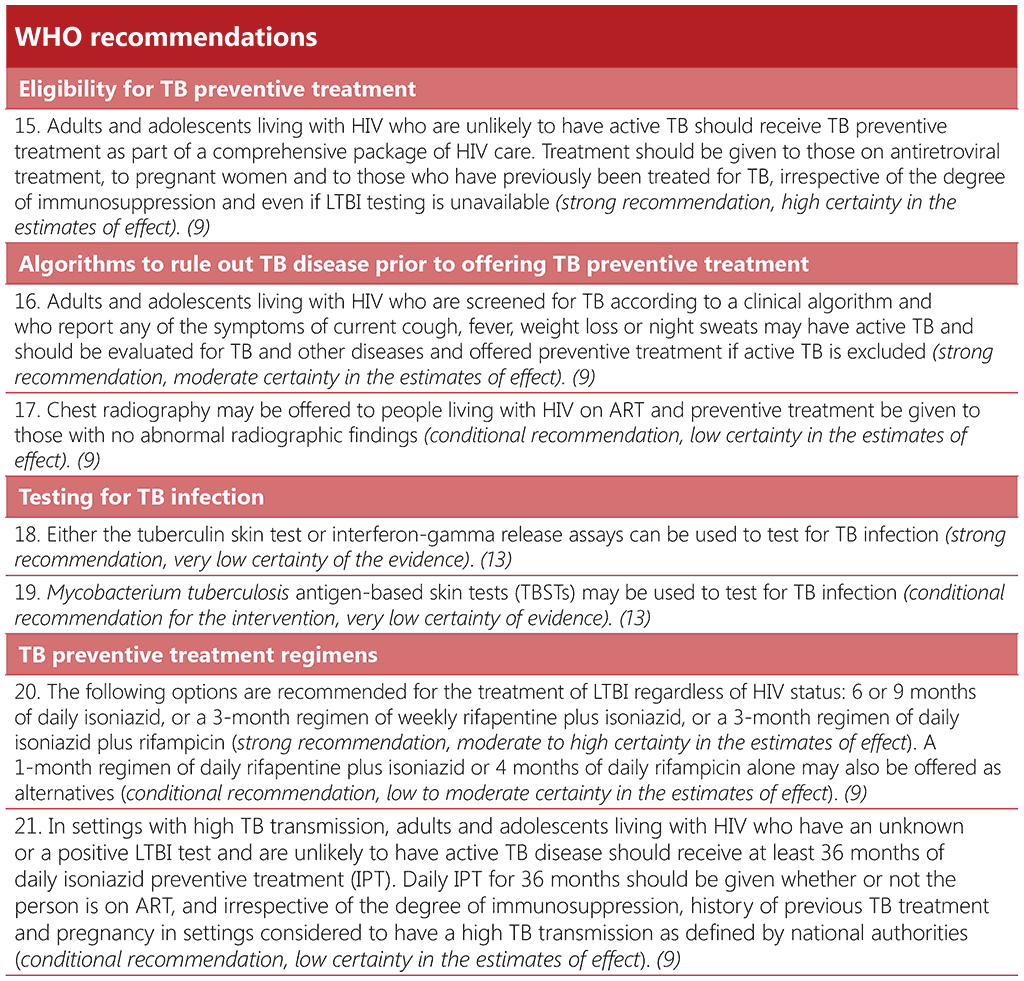
WHO recommends that all adults and adolescents with HIV who are unlikely to have TB disease should receive TPT as part of a comprehensive package of HIV care. This includes those receiving ART, pregnant women and those who have previously been treated for TB; it applies irrespective of the degree of immunosuppression and even if TB infection testing is unavailable (9). Whilst there is no evidence to date on the utility of repeated courses of TPT, and WHO does not have any recommendations on this approach, a repeat course of TPT should be considered among people living with HIV who have previously completed a course of TPT and have been thereafter a household or close contact of a person with TB (113).
3.5.1.2 Ruling out TB disease prior to offering TB preventive treatment
Offering TPT to someone who has TB disease can delay the resolution of disease and may favour the emergence of drug resistance. Thus, excluding TB disease before initiating TPT is one of the critical steps in the TPT care pathway. Table 3.5 summarizes the key steps in ruling out TB disease prior to starting TPT in people living with HIV.
Screening for TB using a standard set of signs and symptoms has multiple advantages. The W4SS – current cough, fever, weight loss or night sweats – is useful for ruling out TB disease among people living with HIV, regardless of ART use. In many settings, it has high sensitivity and a high negative predictive value, and it is a straightforward intervention; it can be implemented during any clinical encounter and repeated as often as necessary, without the need for special equipment. Where available, additional tests such as chest radiography, CRP or mWRD may also be used to improve screening accuracy (76). These tests are described in more detail in Section 3.1.
WHO specifically recommends that chest radiography may be offered, if available, for people who are receiving ART. If there are no TB symptoms or abnormal radiographic findings, TPT should be considered. The use of chest radiography together with the W4SS is likely to increase the confidence of health providers given the very high sensitivity of the combination (with less chance of missing TB disease). This may reduce potential provider concerns around the development of drug resistant-TB resulting from inadvertent treatment of TB disease with a TPT regimen. However, chest radiography provides only a marginal gain in accuracy for ruling out TB disease, as compared with the W4SS alone, and will result in more screen positive results, which would require more investigations for TB and other illnesses. The addition of chest radiography to symptom screening may also present logistical difficulties and increase the cost to programmes and individuals, leading to missed opportunities to give TPT to people who could benefit from it (113). Chest radiography should only be added as an additional investigation if it does not pose a barrier to the provision of preventive treatment for people living with HIV (113).
If a person living with HIV has a positive screening test for TB with any of the WHO-recommended screening tools, but TB disease is ruled out after subsequent diagnostic investigations, the individual should also receive TPT. Thus, collaboration between TB and HIV services is critical to minimize attrition across the diagnostic and prevention cascades of care, and to close the gap in TPT coverage.
Algorithm 6: Algorithm to rule out TB disease in people living with HIV
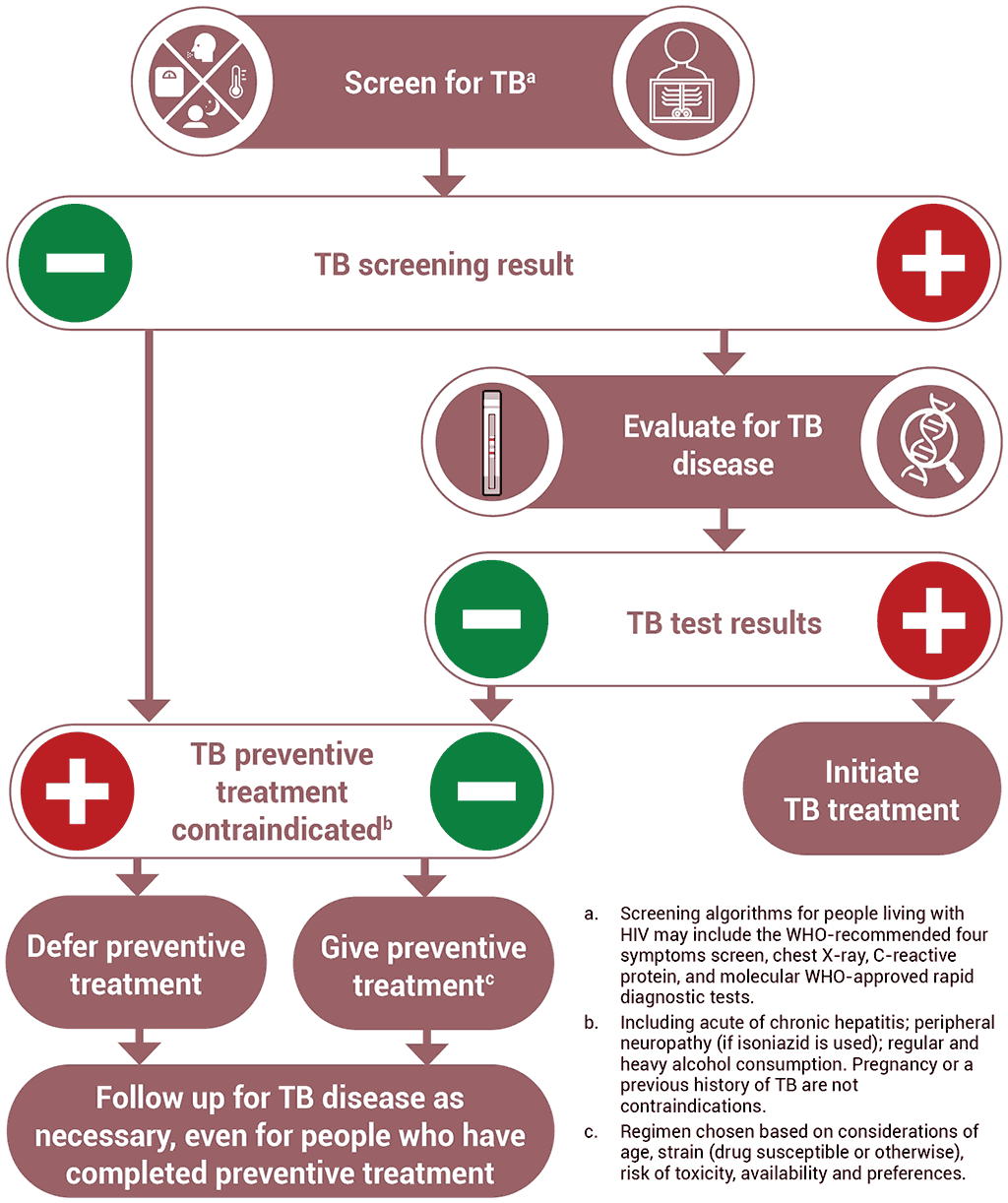
Table 3.5. Key considerations for assessment of eligibility for TPT in people living with HIV (113)
3.5.1.3 Testing for TB infection
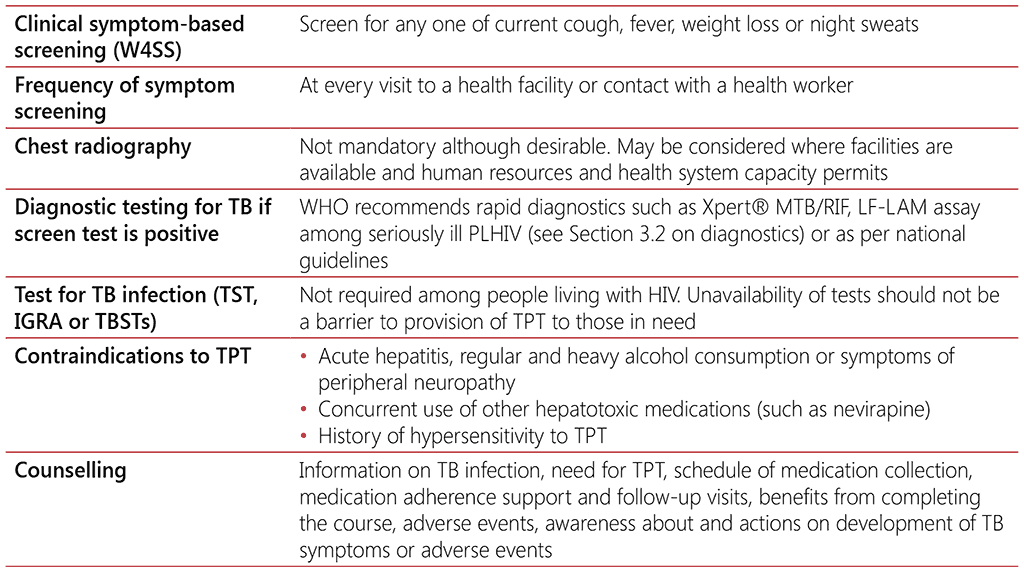
Testing for TB infection prior to initiating TPT is not required for people living with HIV. People living with HIV who are on ART benefit from TPT regardless of whether they test positive or negative for TB infection (114). People living with HIV who are not on ART and who test positive for TB infection are shown to benefit more from TPT than those with a negative test (73); however, testing for TB infection should not be a requirement for initiating TPT among people living with HIV, particularly in countries with high TB incidence, given that the benefits of treatment (even without testing) clearly outweigh the risks (9). Similarly, unavailability of TB infection testing should not constitute a barrier to providing TPT for people living with HIV.
There is no gold standard to diagnose TB infection, however, WHO recommendations have been formulated for three types of tests for TB infection: the tuberculin skin test (TST), the interferongamma release assay, and newer M. tuberculosis antigen-based skin tests (9, 12, 13). The TST is a point-of-care test that involves intradermal injection of purified protein derivative (PPD), a crude mixture of mycobacterial antigens, which stimulates a hypersensitivity reaction that causes an induration at the injection site within 48 to 72 hours but has low specificity among people living with HIV. Recurrent global shortages and stockouts of PPD reduce prospects for the scale-up of this test and may pose a further barrier to the programmatic management of TPT in people living with HIV.
In contrast, IGRAs are in vitro tests that measure the release of interferon-gamma (IFN-γ) following stimulation with two M. tuberculosis-specific antigens: the early secretory antigenic target 6 kDa protein (ESAT-6) and culture filtrate protein 10 (CFP-10). IGRAs are more expensive than TSTs and require specialized facilities and trained personnel (115). Both TST and IGRA require the person to mount an immune response to M. tuberculosis to work properly, thus a negative result does not rule out TB infection, especially among people living with HIV who may not mount an immune response to M. tuberculosis antigens. TST and IGRA could remain positive even after successful completion of TPT. Therefore, results of TST and IGRA should not be used to assess the efficacy of TPT. Since 2022, WHO also recommends the use of newer TBSTs, including Cy-Tb, Diaskintest® and C-TST (formerly known as ESAT6-CFP10 test). The TBSTs are point-of-care skin tests that measure the response to the ESAT-6 and CFP-10 antigens, combining the simple, point-of-care based platform of the TST with the specificity of IGRAs (13).
3.5.1.4 TB preventive treatment regimens
TB preventive treatment for an infection with strains presumed to be drug-susceptible broadly falls into two categories: (i) isoniazid monotherapy for at least 6 months, and (ii) rifamycinbased shorter preventive treatment regimens. Isoniazid preventive therapy has been the most widely used type of TB preventive treatment, but the shorter duration of rifamycin regimens presents a clear advantage (9). The WHO consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment outlines 9H, 6H, 4R, 3HP, 3HR, 1HP and 36H as TPT options for use across all disease burden settings and target populations including people living with HIV, as summarized in Table 3.6 (9). However, a potential challenge with rifamycin-based TPT regimens among people living with HIV is drug–drug interactions. The choice of TPT regimen will depend on availability of appropriate formulations and considerations for age, safety, drug–drug interactions and adherence. Preventive treatment for people who have been in close contact with a person with MDR-TB requires a different regimen using a fluoroquinolone or other second-line agents (9). Further guidance on MDR-TB preventive treatment is due to come out in 2024.
Table 3.6. TB preventive treatment regimens
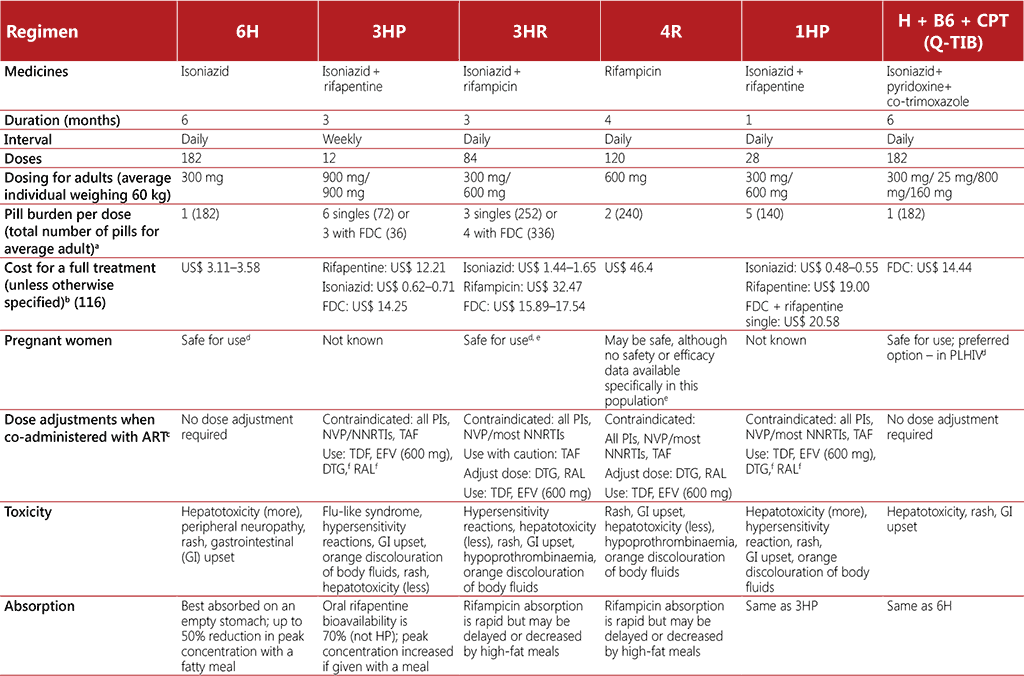
Note: B6 = pyridoxine, CPT = co-trimoxazole, DTG = dolutegravir, EFV = efavirenz, FDC: fixed dose combination, H = isoniazid, LPV/RTV = lopinavir+ritonavir, NNRTI = non-nucleoside reverse transcriptase inhibitor, NVP = nevirapine, P = rifapentine, PIs = protease inhibitors, R = rifampicin, RAL = raltegravir, TAF = tenofovir alafenamide, TDF = tenofovir disoproxil fumarate
a Average available adult formulations: H-300 mg, R-300 mg/150 mg, P-300 mg.
b Prices as per the Global Drug Facility Medicines Catalog, March 2023 (116). Advice on planning orders, including details on the cost of regimens, can also be found on the GDF website: https://www.stoptb.org/buyers/plan-order.
c For women living with HIV (as well as HIV-negative) receiving rifamycin-based TPT and oral contraceptives, consider additional barrier contraception methods to prevent pregnancy.
d One randomized trial has shown increased risk of poor birth outcomes for mothers taking isoniazid during pregnancy; however, several other studies have shown benefits of IPT, hence caution is required.
e Bleeding attributed to hypoprothrombinaemia has been reported in infants and mothers following the use of rifampicin in late pregnancy. Vitamin K is recommended for both the mother and the infant postpartum if rifampicin is used in the last few weeks of pregnancy (US Food and Drug Administration).
f Indicates that drug interaction has been studied in adults and not children; applies to adults taking DTG or RAL only.
The efficacy, safety and convenience of repeated treatment with shorter rifapentine regimens is being studied in people living with HIV in such settings. Findings from one study have shown that a second round of TPT did not provide additional benefit to persons receiving antiretroviral therapy (117). The definition of a high TB transmission setting should be established by the national authorities⁴ (see also Definitions).
Long-term treatment with high-dose isoniazid may cause peripheral neuropathy, which develops secondary to a deficiency of vitamin B6 (pyridoxine) during therapy. Individuals at risk of peripheral neuropathy include people living with HIV as well as those with chronic malnutrition, renal failure or diabetes, or those who are pregnant or breastfeeding. All people living with HIV should receive pyridoxine while taking isoniazid, to prevent peripheral neuropathy. Preventive and therapeutic doses of pyridoxine are available through the Stop TB Partnership Global Drug Facility (GDF). National programmes may consider the use of a triple pill combination of isoniazid, co-trimoxazole and pyridoxine for people living with HIV, instead of an isoniazid-only regimen, available at a discounted price through the GDF (116).
TPT regimens and antiretrovirals
The 3HP regimen can be administered to individuals receiving efavirenz-based antiretroviral regimens without dose adjustment, according to a study of pharmacokinetics (118). The 1HP or 3HP regimens for TPT are not recommended for people receiving PIs or NVP because of the risk of HIV virological failure. Administration of rifapentine with raltegravir was found to be safe and well tolerated (119). Results from a Phase 1/2 trial of 3HP and dolutegravir in adults with HIV reported good tolerance and viral load suppression, no adverse events of Grade ≥3 related to 3HP, and it did not indicate that rifapentine reduced dolutegravir levels sufficiently to require dose adjustment (120). There is a continued need for studies of the pharmacokinetics of 3HP concomitantly with other medicines, particularly ART.
In terms of timing of TPT start, preliminary evidence from another Phase 1/2 trial (DOLPHIN TOO) supports the immediate start of TPT among ART-naïve people starting a dolutegravir based regimen. 3HP administered to 50 persons with HIV who were ART-naïve and started on dolutegravir-containing ART showed high rates of viral suppression comparable with 6H and no difference in GRADE 3 or 4 adverse events ([Ethel Weld], [Johns Hopkins University], DOLPHIN-TOO: Oral abstract presented at The Union World Conference on Lung Health, [2023]).
Preventive treatment for MDR-TB
The capacity of programmes to provide preventive treatment for MDR-TB should be carefully planned for. Providing preventive treatment for MDR-TB requires that all the necessary resources are in place. Considerations should include the ability to identify people who may benefit from MDRTB preventive treatment, the capacity to rule out TB disease, to perform quality-assured testing for drug susceptibility (in the presumed index patient), to deliver the necessary medications and to monitor closely for adverse events and for the emergence of active disease. The choice of preventive treatment for MDR-TB is discussed further in the WHO consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment (9) and the accompanying operational handbook (113).
Liver function tests
There is insufficient evidence to support mandatory or routine liver function tests (LFTs) at baseline (121), and perhaps the benefit of TPT without LFTs would likely outweigh harm, particularly with a less hepatotoxic regimen. However, where feasible, baseline testing is strongly encouraged for individuals with risk factors – such as a history of liver disease, regular use of alcohol, chronic liver disease, HIV infection, age over 35 years and in pregnancy or immediate postpartum period (within 3 months of delivery). In individuals having abnormal baseline LFT results, sound clinical judgement is required to determine if the benefit of TPT outweighs the risk of adverse events. These individuals should be tested routinely at subsequent visits.
Subpopulation considerations
TPT among pregnant women living with HIV: pregnant women living with HIV are at higher risk of TB during pregnancy and postpartum, which can have severe consequences for both the mother and the infant (122, 123). Pregnancy should not prevent women living with HIV from receiving TPT. Preventive treatment should be started during the antenatal and postnatal periods, provided it is given with due care. There are limited data on the efficacy and safety of rifapentine during pregnancy. WHO currently recommends 6 months of isoniazid regimen as TB preventive treatment for pregnant women living with HIV. The triple pill combination of isoniazid+co-trimoxazole+B6 may be the preferred option for TPT among pregnant and postpartum women living with HIV until more safety data on the use of rifapentine-based shorter regimens are available.
TPT among people who use drugs: people who use drugs (PWUD) have a higher prevalence of TB infection and incidence of TB disease, regardless of HIV status, when compared to people who do not use drugs (124). Isoniazid preventive therapy is safe to use among PWUD, if acute hepatitis or heavy alcohol consumption are ruled out, whilst careful monitoring for liver toxicity is important (125). WHO recommends methadone or buprenorphine for treating opioid dependence (126). Rifampicin is known to reduce exposure to OAMT such as methadone and buprenorphine (127), which may result in opiate withdrawal syndrome. Rifapentine has not been systematically studied among PWUD. People taking 1HP, 3HP, 3HR or 4R with ART and OAMT should be closely monitored for signs of opiate withdrawal and other adverse events, and doses of methadone or buprenorphine may need to be increased accordingly, to lessen the risk of withdrawal. Drug use should not be a reason to deny someone access to TPT, and healthcare providers should proactively manage drug– drug interactions for PWUD safely, as well as provide treatment and adherence support (128).
Scale-up of TB preventive treatment
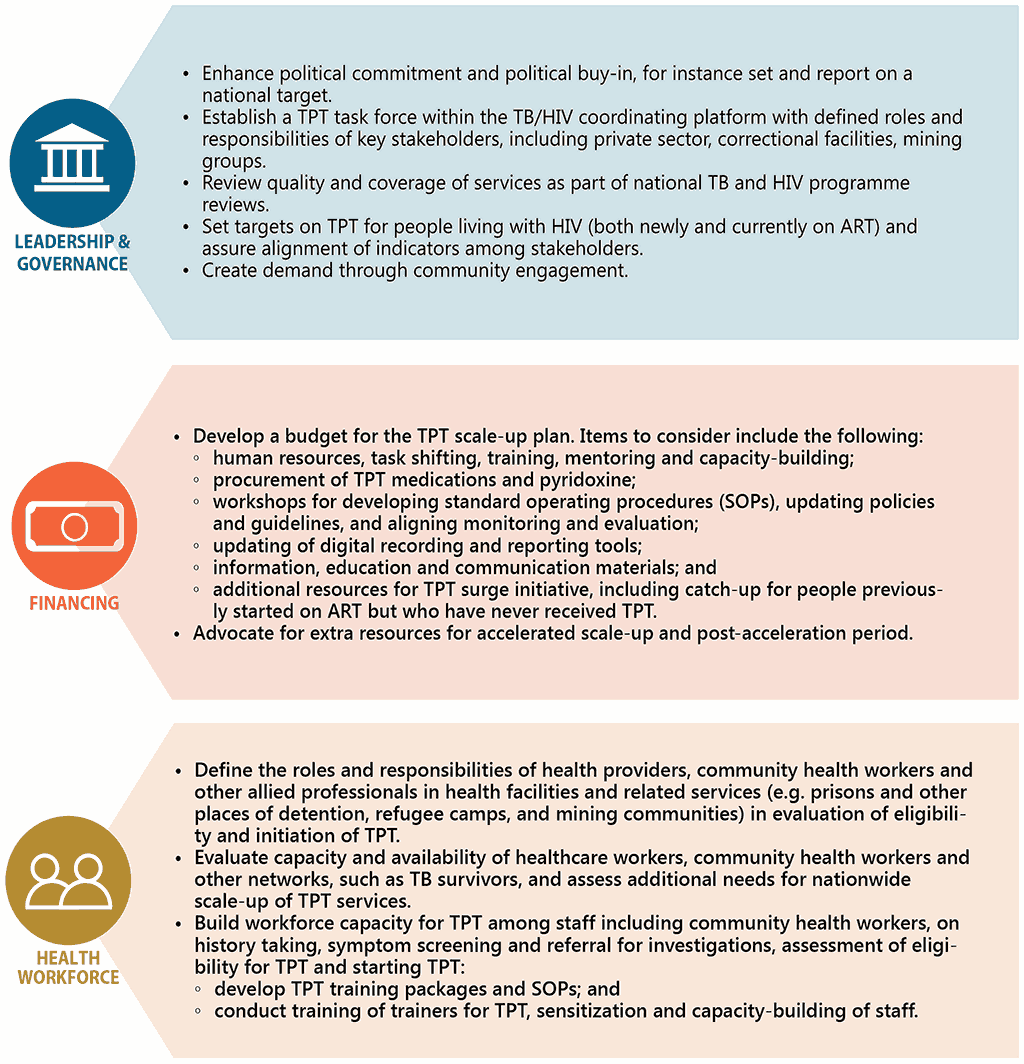
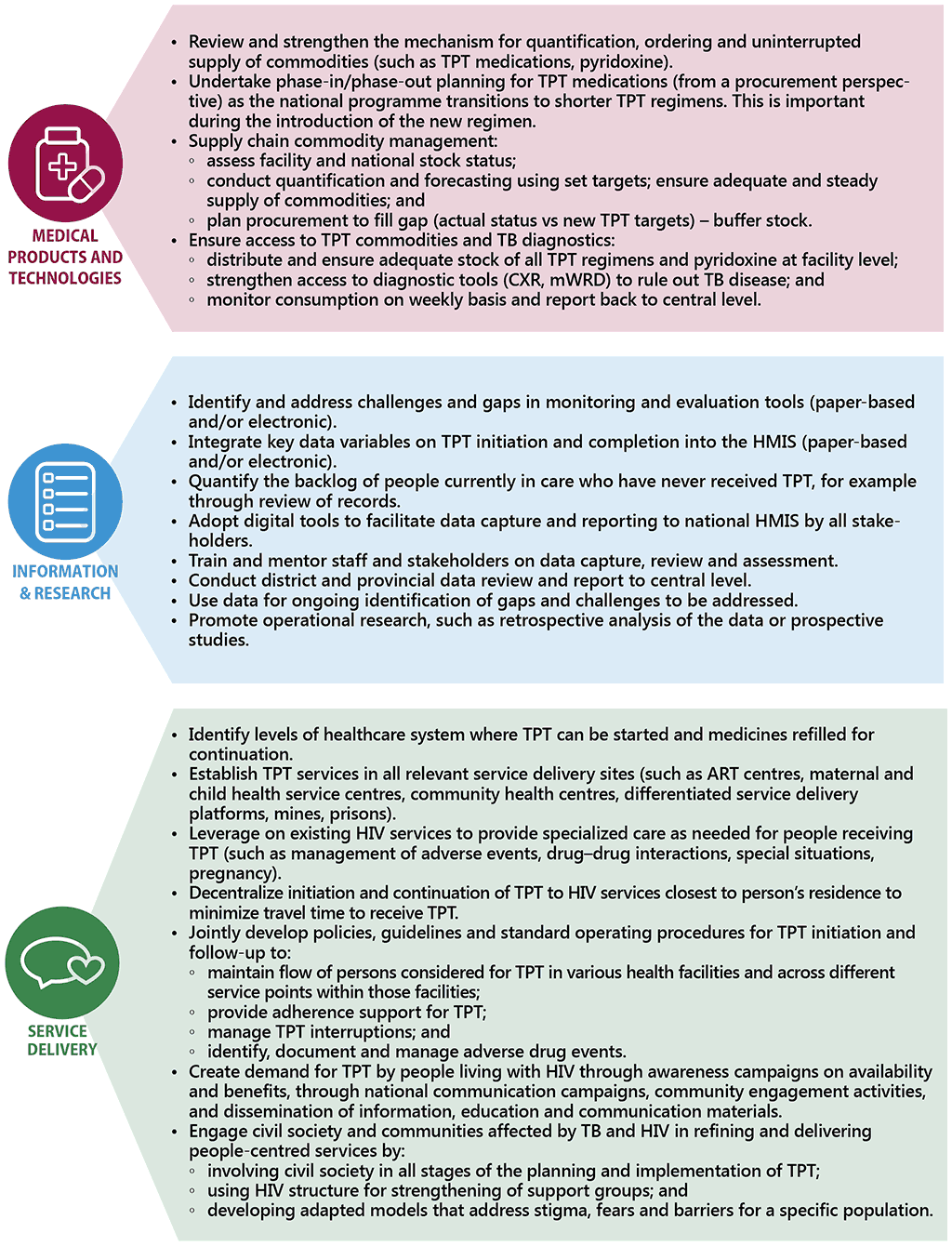
In recent years, as part of efforts to meeting the commitments made at the first UN High-level Meeting on the Fight Against Tuberculosis, there has been considerable scale-up of TPT among people living with HIV. Previously, countries prioritized TPT among individuals newly enrolled on ART. However, given that all people living with HIV are eligible for TPT, countries are now beginning to conduct catch-up TPT campaigns or TPT surge initiatives to include people already on ART who have not yet received TPT. Whilst there is need for careful resource planning for such initiatives, these actions have proven to be effective in gaining political support and engaging stakeholders across multiple sectors (129). Fig. 3.2 draws experience from countries in scaling up TPT and outlines the enablers for scale-up in line with health system building blocks.
Fig. 3.2 Key enablers to scaling up TPT among people living with HIV
3.5.2 Infection prevention and control
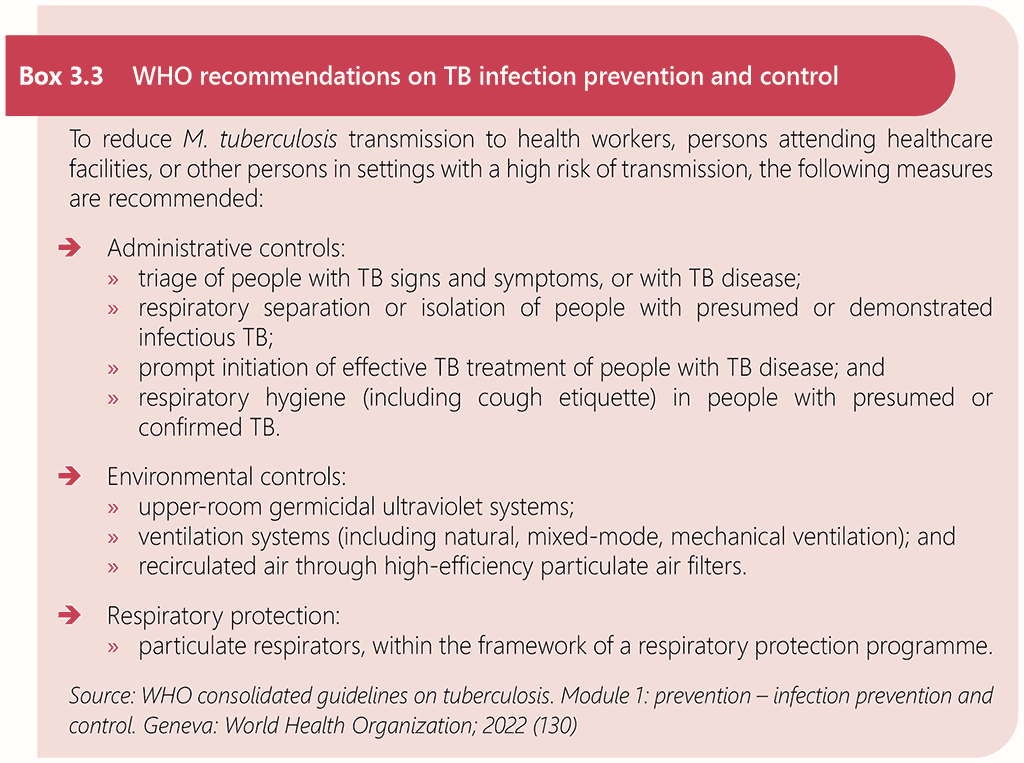
Comprehensive infection prevention and control (IPC) measures are essential to prevent TB transmission in clinical settings that provide services for people living with HIV (130). There are no separate infection prevention and control measures that are specific to HIV-associated TB. The WHO consolidated guidelines on tuberculosis. Module 1: prevention – infection prevention and control (130) provide recommendations on preventing the transmission of TB in health care and other congregate settings, through administrative controls, environmental controls and respiratory protection measures. The WHO Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level (131) provide additional detail on IPC measures to prevent transmission of infectious diseases that apply to all healthcare settings. Box 3.3 summarizes WHO recommendations on TB infection prevention and control.
Healthcare facilities and congregate settings can present a risk for acquiring TB (including MDR-TB) for people living with HIV, as well as for healthcare workers. At facility level, measures to reduce TB transmission include administrative controls, environmental controls and respiratory protection, which are aimed at generally reducing exposure to M. tuberculosis for clients, healthcare workers, prison staff, police and any other persons visiting or working in congregate settings (132).
To minimize time spent in healthcare facilities, community-based or home-based treatment support is recommended over health facility-based treatment support or unsupervised treatment (15). People with HIV and TB and their communities should be trained on TB transmission, infection prevention and control, and cough etiquette to reduce the risk of TB transmission in healthcare facilities, in congregate settings and within their own homes.
All measures should be taken to ensure that people working in healthcare facilities and congregate settings have access to prevention measures, including HIV prevention interventions, and ART and TPT for workers who are living with HIV. Healthcare workers should have access to acceptable, confidential and quality-assured HIV testing services. Even when they are on ART, healthcare workers with HIV will remain at higher risk of developing TB, and transfer of their clinical responsibilities to sites that have the least risk of TB transmission, as well as regular TB screening, should be considered to mitigate this risk. Similarly, healthcare workers with TB disease should be temporarily relocated from HIV care facilities.
Implementation of TB infection prevention and control measures requires managerial oversight at national, subnational and facility levels, which includes establishing TB infection prevention and control committees at all levels; developing a plan preferably incorporated into a broader infection prevention and control plan; appropriate health facility design and use; surveillance of TB disease among healthcare workers; an advocacy and communication strategy; monitoring and evaluation; and operational research (130). Periodic evaluation of infection prevention and control practices is essential to ensure that appropriate measures are in place. Facility-level assessment of TB infection prevention and control should be incorporated into the routine supervisory activities of all health facilities which provide care for people living with HIV. A standardized checklist for periodic evaluation of infection prevention and control practices can serve as a tool for such an assessment and can help measure progress over time. An example of a checklist that can be adapted by countries to suit the context can be found in Annex 3. To reduce the transmission of TB to the family and the community, key information, education and counselling should also be provided to the individual and family members. This should include advice on cough etiquette, sleeping alone, avoiding congregate settings and spending as much time as possible outdoors where feasible, until no longer infectious (130).
4 A setting with a high frequency of individuals with undetected or undiagnosed active TB, or where individuals with infectious TB are present and there is a high risk of TB transmission. TB is most infectious when it is untreated or inadequately treated. Spread is increased by aerosol-generating procedures and by the presence of highly susceptible individuals.
 Retour
Retour