Enlaces transversales de Book para 1.2 TB infection tests that are currently recommended by WHO
In 1908, Mantoux performed the first intradermal test for TB infection using killed M. tuberculosis (9). The basic principles of this test have not changed in more than 100 years, making the tuberculin skin test (TST) one of the oldest tests still in clinical use. The skin test that is in widespread use today comprises the intradermal injection of a purified protein derivative (PPD) from heat-killed cultures of M. tuberculosis, with measurement of the resultant induration 48–72 hours later. Although standardized, this PPD material comprises a heterogeneous mixture of TB antigens, which results in significant cross-reactivity with other M. tuberculosis complex strains – notably bacille Calmette-Guérin (BCG) and nontuberculous mycobacteria (10–12), reducing specificity.
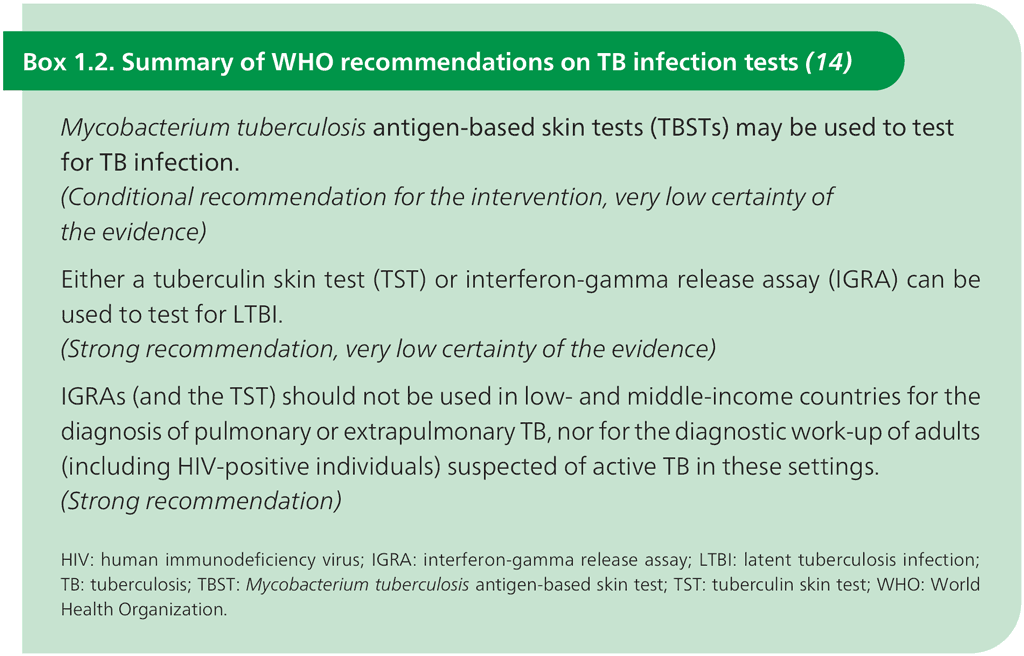
Over the past 20 years, more specific tests for TB infection have been developed; these primarily use the early secretory antigenic 6 kDa (ESAT-6) protein and culture filtrate protein 10 (CFP-10), which are antigens that are found in M. tuberculosis but are not present in BCG or in most species of nontuberculous mycobacteria (13). Testing for these two antigens has been performed using interferon-gamma release assays (IGRAs) on a blood sample. These tests measure interferon-gamma production from whole blood or from circulating lymphocytes after in vitro overnight incubation in the presence of ESAT-6 and CFP-10. More recently, intradermal skin tests targeting the same two antigens (M. tuberculosis antigen-based skin tests, TBSTs) have become commercially available. Since 2022, WHO has recommended the use of the new TBSTs as a way to test for TB infection, alongside older recommendations on IGRAs and TST (Box 1.2).
 Reacción
Reacción