Enlaces transversales de Book para 2.6.1 Phenotypic DST
Treatment of TB has undergone significant changes over recent years, with new drugs and regimens recommended; hence, the definitions for DR-TB have been revised accordingly. The updated pre-extensively drug-resistant TB (pre-XDR-TB) definition is “TB caused by M. tuberculosis strains that fulfil the definition of MDR/RR-TB and are also resistant to any FQ”, and the updated XDR-TB definition is “TB caused by M. tuberculosis strains that fulfil the definition of MDR/RR-TB and that are also resistant to any FQ and at least one additional Group A drug (i.e. BDQ or LZD)” (23). These changes have important implications for Member States, particularly for the scaling-up of the detection of resistance to FQs and BDQ. In addition, there is an increasing demand for DST for other new and repurposed drugs.
In 2018, WHO updated the CCs used in phenotypic DST to include the new and repurposed drugs for existing methods (24). In 2023, the CCs for Pa and cycloserine were established (Web Annex B). The updated manual on culture-based DST for medicines used in the treatment of TB is included at Web Annex C. The updated DST manual describes the WHO recommendations for phenotypic and genotypic DST for anti-TB medicines. Also, the manual provides CCs to be used in solid and liquid media for phenotypic DST of medicines used to treat drug-susceptible TB and DR-TB, sources of pure powders for phenotypic DST, detailed methods for preparing drug-containing media, interpretation and reporting of results, and quality control (QC).
The new definition of XDR-TB requires DST results for FQs, BDQ and LZD; thus, testing for resistance to BDQ and LZD has become a priority, particularly testing for resistance to BDQ. Phenotypic DST for BDQ and LZD can be performed using either the MGIT or Middlebrook 7H11 media. Lyophilized vials containing BDQ powder for use with MGIT are available from Becton Dickinson, and are expected to be listed in the Global Drug Facility (GDF) catalogue in 2024. In addition, the BDQ pure drug substance for use in phenotypic DST is provided free through the US National Institutes of Health (NIH) HIV Reagent Program (25); however, courier costs need to be covered. An information note that explains the request process is also available (26). In 2024, the HIV Reagent Program will also provide DLM and Pa. LZD powder is available from Sigma (PZ0014–5MG) or Cayman Chemical (CAS 65800–03–3). The updated DST manual in Web Annex C provides specific details on the sources of the drug powders used for phenotypic DST.
CCs for the rifamycins and INH were also reviewed, and findings were published in 2021 (27). The CCs for INH have remained the same, whereas those for RIF in MGIT and 7H10 have been revised downwards, from 1.0 mg/L to 0.5 mg/L. These revisions are expected to reduce but not eliminate discordance between genotypic and phenotypic DST results, and laboratories are advised to implement these changes immediately. For MGIT, this can be achieved by reconstituting the lyophilized RIF from the BD SIRE kit in 8 mL (instead of 4 mL) of sterile distilled or deionized water. The sources of other pure drug substances for DST are described in Table 5 in Web Annex C.
The latest CCs for all drugs are listed in Tables 2.2 and 2.3; they were adapted from Tables 2 and 3 of Web Annex C.
Table 2.2. CCs for first-line medicines recommended for the treatment of drug-susceptible TB
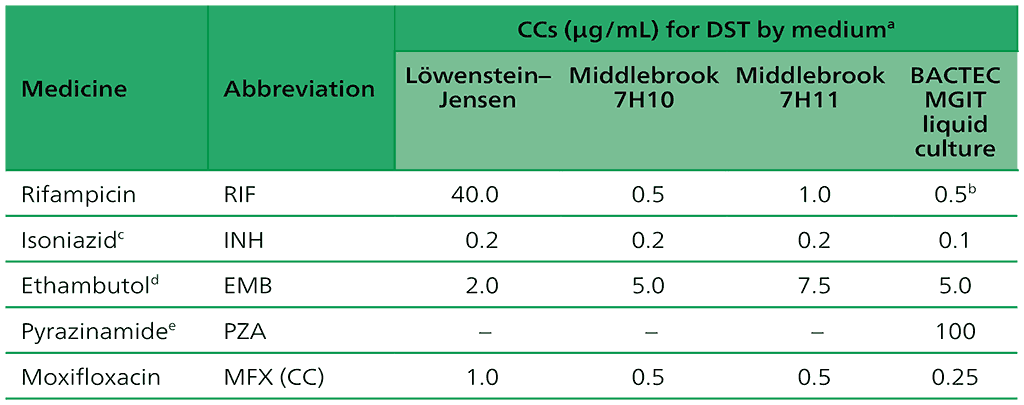
CC: critical concentration; DNA: deoxyribonucleic acid; DST: drug susceptibility testing; INH: isoniazid; LPA: line probe assay; MGIT: Mycobacterial Growth Indicator Tube; MTBC: Mycobacterium tuberculosis complex; NAAT: nucleic acid amplification test; PZA: pyrazinamide; TB: tuberculosis.
a The use of the indirect proportion method is recommended. Other methods using solid media (e.g. the resistance ratio or absolute concentration) have not been adequately validated for anti-TB agents.
b The detection of rifampicin resistance using the BACTEC MGIT 960 system has limitations and cannot detect clinically significant resistance in certain isolates. The detection of resistance-conferring mutations in the entire rpoB gene using DNA sequencing may be the most reliable method for the detection of rifampicin resistance.
c People with MTBC isolates that are resistant at the CC may be effectively treated with high-dose isoniazid. Formerly, a higher concentration of INH (0.4 µg/ml in MGIT) was used to identify strains that may be effectively treated with a higher drug dose. However, molecular patterns of INH resistance may be more reliable for predicting patient outcomes than phenotypic DST. To date, no clinical breakpoint concentration has been established for INH.
d All phenotypic DST methods for ethambutol produce inconsistent results. Phenotypic DST is not recommended.
e BACTEC MGIT 960 liquid culture method for PZA susceptibility testing is reportedly associated with a high rate of false positive resistance results. Careful inoculum preparation is essential for performing PZA testing reliably. The Genoscholar PZA-TB LPA is the only high complexity hybridization NAAT recommended for PZA. The detection of resistance-conferring mutations in the pncA gene using DNA sequencing may be the most reliable method for the detection of PZA resistance although there is emerging evidence of non-pncA mutational resistance to PZA.
Source: adapted from Table 2 in Web Annex C.
Table 2.3. CCs and clinical breakpoints for medicines recommended for the treatment of MDR/RR-TB (adapted from Table 3 in Web Annex C)
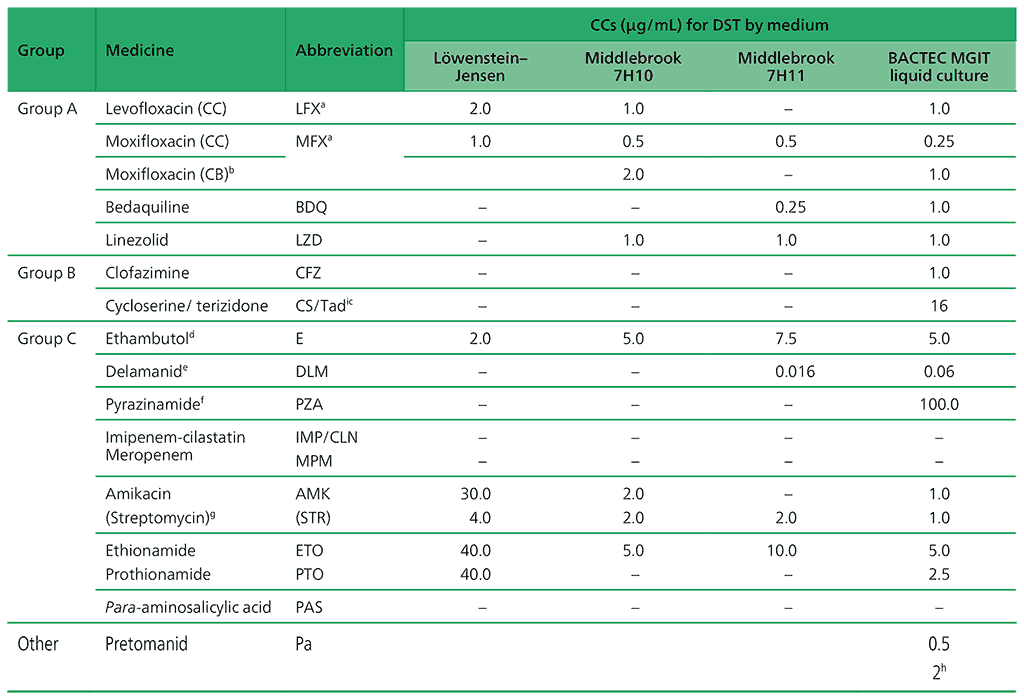
ATU: area of technical uncertainty; CB: clinical breakpoint; CC: critical concentration; DST: drug susceptibility testing; LJ: Löwenstein–Jensen media; MDR/RR-TB: multidrug- or rifampicin-resistant tuberculosis; MGIT: Mycobacterial Growth Indicator Tube.
a LFX and MFX CCs for LJ established despite very limited data.
b CB concentration for 7H10 and MGIT apply to high-dose MFX (i.e. 800 mg daily).
c The CC for CS may be used as a surrogate for terizidone resistance.
d DST not reliable and reproducible. DST is not recommended.
e DLM should be stored away from light and heat, as per the manufacturer’s materials safety data sheet.
f PZA is only counted as an effective agent when DST results confirm susceptibility in a quality-assured laboratory.
g AMK and STR are only to be considered in case of rescue regimens or individualized treatment, and only if DST results confirm susceptibility.
h No growth at 0.5 = susceptible; growth at 0.5 and no growth at 2.0 = susceptible, but with a comment on uncertainty; growth at 2.0 = resistant.
 Reacción
Reacción