Enlaces transversales de Book para A1.2 – Reading of TST or TBST result
Step 1. Seeing the patient
Patients should be seen as quickly as possible after they arrive for the reading of their TST or TBST. Reading of the test takes less than 3 minutes on average (7). If a person must wait a long time simply for the result to be read, this may discourage other contacts in the same household from coming forward for testing or reading, and may discourage the same person from having a re-test, if needed.
Step 2. Re-check symptoms
If the person had non-TB respiratory symptoms at the time the test was administered, then these symptoms should be re-checked at the time of reading. If the symptoms are less severe or have resolved, then this individual can be considered to have “no symptoms” in the algorithm. However, if symptoms have not improved – and particularly if the symptoms have worsened or are suggestive of TB (e.g. fever or night sweats) – the individual should be considered to have symptoms for investigation. In such cases, the result of the TST or TBST should be read and recorded, but the person should be referred promptly for medical evaluation, including chest X-ray (CXR) – if not already done – and other testing as appropriate, regardless of the result of the test for TB infection.
Step 3. Place and position of patient for reading
Reading should be done in a private room (wherever possible), out of view of all other individuals (although family members can attend, as appropriate). For optimal measurement, the patient should be seated with the arm supported.
Step 4. Inspection of injection site
The site of the injection for the TST or TBST should be carefully inspected. If there is blistering, skin breakdown or lymphadenitis these should be recorded because they are considered to be strong positive reactions for all TB infection skin tests.
Erythema or redness is an indicator of potential induration (if erythema is present, then induration may be present). However, erythema does not need to be measured for TST (3), or for Cy-Tb (6), and only needs to be measured for Diaskintest if there is no induration (5). For C-TST, erythema should be demarcated and measured (4).
Induration or firm swelling can be detected visually or by palpation.
If there is no redness and no visible or palpable induration, then the test is negative and the result is marked as “0 mm”. Likewise, if there is erythema but no obvious swelling, and on light palpation there is no evidence of induration, then the induration can be considered negative and “0 mm” can be recorded. If any swelling or induration can be palpated, then this should be demarcated (see Step 5) and measured.
If an mHealth QC programme is in place, then photographs of the injection site should be taken.
Step 5. Demarcation of induration
For TST, Cy-Tb and Diaskintest, the induration is measured (3, 5, 6). For C-TST, both induration and erythema are measured, and the largest of the two is used for clinical management (4).
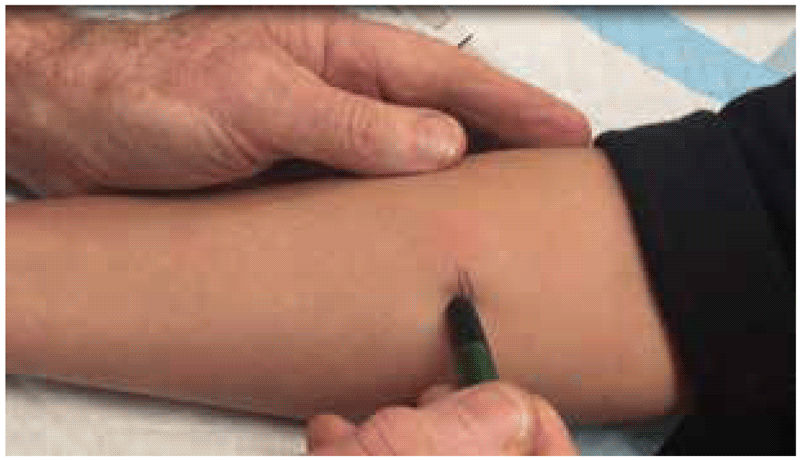
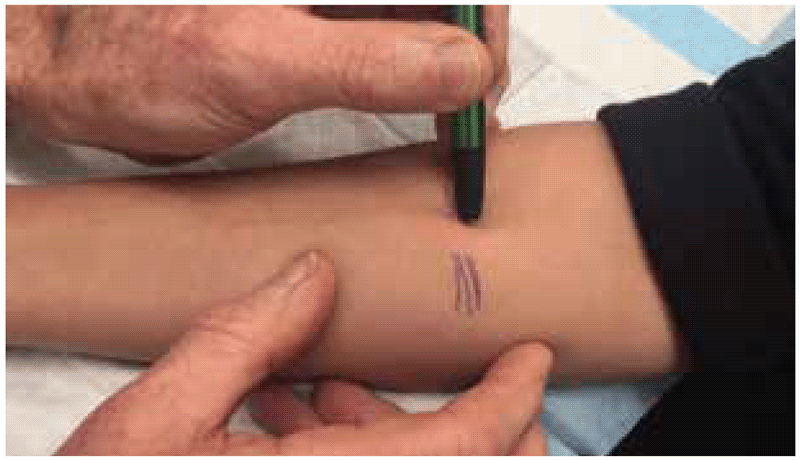
To demarcate the edges of the induration, the ballpoint pen method can be used (8). With this method, a ballpoint pen tip is pushed gently against the skin at a 45-degree angle towards the site of the injection. If there is a firm and distinct induration, the ballpoint pen tip will consistently stop at the margins. This procedure is repeated several times from different directions around the injection site. If there is no visible or palpable induration, it is not necessary to use a ballpoint pen; the result can be marked as “0 mm”.
Large reactions can be painful, and it is not necessary to insist on demarcating and measuring large painful indurations. These can be simply marked as “greater than 15 mm” or “greater than 20 mm” and any blistering or skin necrosis noted.
Step 6. Measurement

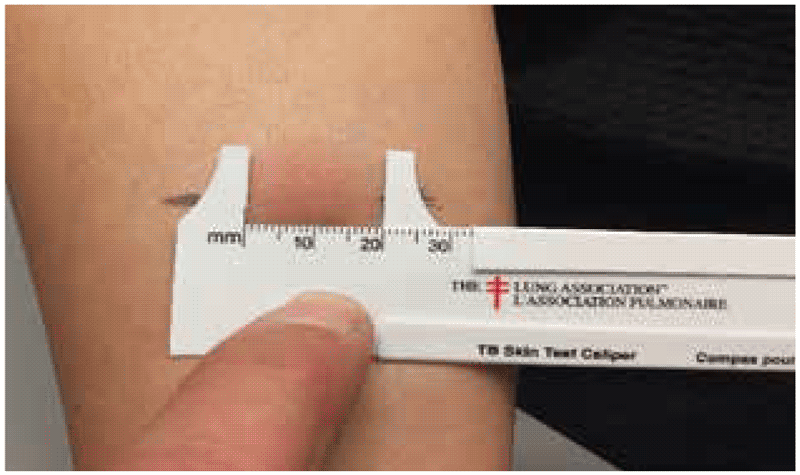
Once the edges of the induration (or, in the case of C-TST, the erythema) have been demarcated, then the diameter of the induration should be measured. For Cy-Tb and TST, the transverse diameter is measured and recorded in millimetres (3, 6). For the C-TST, the transverse and longitudinal diameters of erythema and induration are measured, and the average of each is recorded (4). The largest of these two will be taken as the clinically relevant information. For the Diaskintest, the largest diameter of the induration in any dimension is recorded (5).
Measurement of the size of reaction is often subject to rounding error, because readers tend to group readings at 5, 10 and 15 mm. To avoid this error, it is a good practice to use machinist calipers or tailor calipers.
Step 7. Post-TST care
If the patient has blistering or skin breakdown, then it is important to prevent secondary infection. The area should be carefully cleaned and covered with a dry dressing. Patients should be instructed not to scratch the area.
Topical steroids should not be used, because these were shown to be ineffective in placebo-controlled trials (9). Subcutaneous injection of steroids under the induration may be more effective, but conservative management with cold compresses and dry dressings to cover the site is usually sufficient and prescription of oral analgesics (aspirin or acetaminophen/ paracetamol).
Step 8. Management of results
If the test is negative (as per Chapter 3 of the main text) and the patient is asymptomatic (or if symptoms are resolving), then the patient can be discharged. In some settings, close contacts will have a second TB infection test 8 weeks after the end of exposure if an initial test is negative – particularly in those with very recent exposure or concomitant viral infection. In this case, an appointment should be made for administration of the second test.
If the test is positive the person should be referred immediately for medical evaluation and CXR. In a well-organized person-centred care cascade, medical evaluation (including CXR) is available at the same site and on the same day. This minimizes delays between the first identification of a contact and the initiation of appropriate therapy for TB disease or infection.
Step 9. Recording and registration
The TST or TBST reading should be recorded in the patient’s medical record or registries (or both); additional information may be recorded, depending on the setting and programme organization.
The following should be recorded:
- date and time of reading;
- product used and lot number;
- size of the induration in millimetres (transverse, maximal or average diameter, depending on the TST or TBST);
- for C-TST, the average diameter of erythema should also be recorded, and for Diaskintest, erythema (but only if there is no induration); for the TST and Cy-Tb, erythema does not need to be recorded;
- presence of blistering, skin necrosis, lymphangitis or lymphadenopathy; and
- for those with positive tests – disposition: date and time of follow-up for medical evaluation and CXR; for those with negative tests, date and time of follow-up appointment, if appropriate.
Certain articles provide useful general information on skin tests for TB infection (10, 11).
 Reacción
Reacción