Enlaces transversales de Book para 3.3.4 Overview of test procedures and management
Administration of the TST or TBST (Mantoux method)
Before administration of the TST or TBST via the Mantoux method (48), the person to be tested should be seated comfortably, with their arm supported. The administrator should take a few minutes to explain the procedure and ensure that the person understands and agrees to testing, and that they can and will return for reading within 48–72 hours. Confidentiality should be maintained, meaning that the testing is performed in a separate room, with only the individual being tested being present (accompanied by family members where appropriate). In cases where a child is administered the test, consent by a parent or guardian is required and the presence of the parent or guardian may be necessary.
The test is administered on the inner aspect of the forearm, about 10 cm below the elbow. This is conventional because the skin surface at that point is fairly flat, making subsequent measurement of induration easier. The spot selected should have no scars, rashes, burns or tattoos.
The tuberculin material is drawn up in the syringe not more than 20 minutes before injection, and the skin is cleaned and allowed to dry. The tuberculin syringe is laid flat on the skin, with the bevel of the needle upward, as shown in an online video (43). The material is injected slowly and should create a small bleb (at least 7 mm in diameter (45)), which will disappear in 15–20 minutes. The material must not be injected subcutaneously because this makes it more difficult to measure the result and can even lead to false negative results. It is not necessary to cover the area with a bandage or to mark the injection site in any way.
After test administration, the individual should be monitored for 10–15 minutes; that is, the individual should be asked to wait, ideally seated. One of the adverse reactions to note is vasovagal fainting, which is to be distinguished from anaphylaxis. Any dizziness or loss of consciousness should be carefully documented, so that, in the future, it is known whether the individual had a vasovagal reaction or anaphylaxis.
Upon discharge, patients should be instructed to keep the injection site clean. If there is significant swelling and itching, they should avoid scratching but can apply a cold compress. Patients can perform all normal activities including bathing or showering. The importance of a return visit within 48–72 hours for reading should be reinforced.
The person who administers the test should record the date, time and position on which forearm the test was administered; the name, manufacturer and lot number of the tuberculin material; and the date on which the vial was opened. Any adverse reactions should be noted.
Reading the resultant reaction
Patients should be instructed to return after 48–72 hours. If an individual fails to present after 48 hours, they can be contacted and reminded to come in the following day. This would allow reading to be performed within the maximum of 72 hours that is optimal for accuracy of all TB infection skin tests (49).
Reading should be done with the patient seated comfortably and the arm supported, so that the skin is relaxed as much as possible. For all tests except C-TST, it is important to measure the induration but not the redness (for C-TST, see below). Induration is best measured using the ballpoint method (50). The margins of the induration can be measured with a ruler or tape measure, but this can lead to a rounding error; hence, reactions are usually grouped at 5, 10 or 15 mm points. More precise measurements can be obtained using simple calipers used by machinists, mechanics or tailors.
The diameter of the induration should be recorded in millimetres, along with the interpretation (positive or negative) and referral for medical evaluation (if positive). Redness can be noted; any blistering should also be noted because this is synonymous with a positive test. If there is significant blistering or skin breakdown, the area should be cleaned and then covered with a dry dressing, simply to protect it from secondary infection. Management with topical steroids is ineffective (51); cold compresses and prescription of oral analgesics (aspirin or acetaminophen/ paracetamol) should be adequate.
Self-reading of test result
Studies have reported low accuracy of self-reading of the TB skin test result. One study noted that 50% of reactions that were read as positive by a trained health professional were interpreted as negative by the patient (22).
Interpretation of TST or TBST
Criteria for interpretation of TST-PPDS have been established in numerous large-scale observational and intervention studies. The sensitivity of different criteria for size of induration was established in a multicentre international study conducted by WHO in the 1950s (34). Numerous studies have demonstrated that the sensitivity of TST can be reduced because of technical problems with the test itself, or biological conditions that impair the cell-mediated immune response (21, 42). These are summarized in Table 3.3. To date, there have been few studies of the new TBSTs to understand the impact of these problems on the sensitivity of these tests; in the absence of such data, it is assumed that sensitivity of the new TBSTs will be affected in the same way. Although technical issues can be identified and corrected, the biological causes of reduced sensitivity are more difficult to correct, so should be considered carefully when interpreting a negative TB infection skin test result.
Table 3.3. Potential causes of false negative TB infection skin testsa
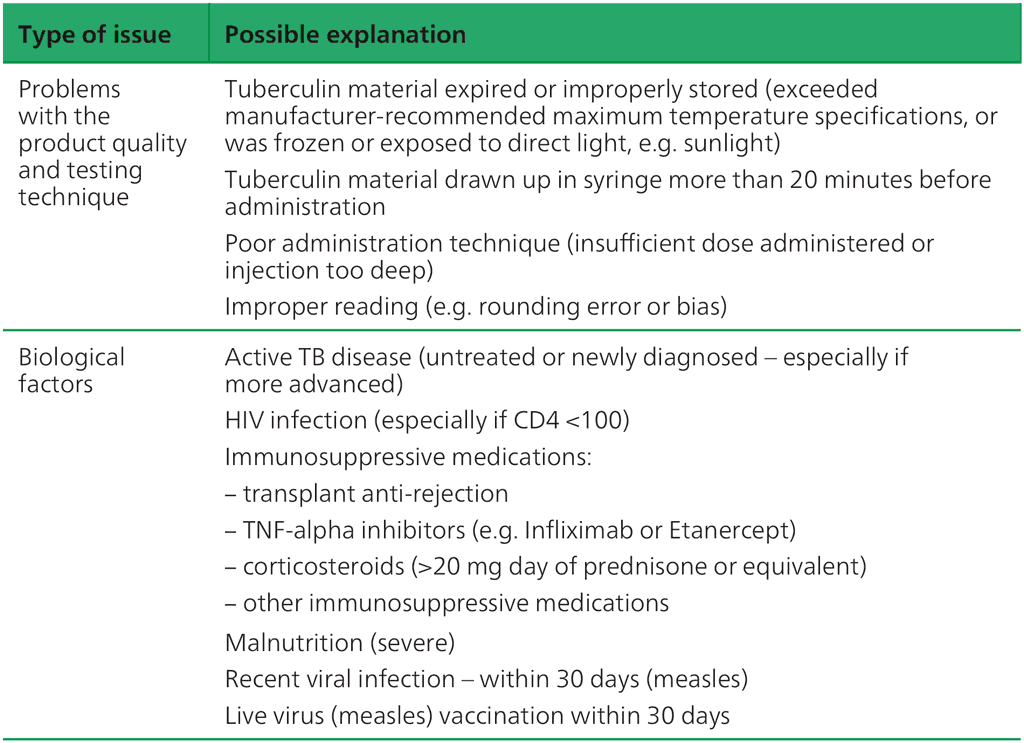
HIV: human immunodeficiency virus; PPDS: standard purified protein derivative; TB: tuberculosis; TBST: Mycobacterium tuberculosis antigen-based skin test; TNF: tumour necrosis factor; TST: tuberculin skin test.
ᵃ All studies used TST (PPDS), but it is assumed that these problems would lead to similar reductions in sensitivity of TBSTs
Many of the antigens found in M. tuberculosis are also present in nontuberculous mycobacteria (e.g. M. avium) and the BCG vaccine. These antigens that are not specific to M. tuberculosis commonly cause false positive reactions to TST. Since the likelihood of prior BCG vaccination or exposure to nontuberculous mycobacteria varies among different populations and clinical situations, the specificity of TST also varies, and is suboptimal in many populations (42). Hence, the criteria to define a positive TST varies according to clinical and epidemiological risk factors (see Table 3.1 above).
Specificity is of much less concern for the IGRAs, or for the new TBST, because these tests measure immune reactions to only two antigens (ESAT-6 and CFP-10) which are much more specific to M. tuberculosis (13). These antigens are present in some nontuberculous mycobacteria such as M. kansasii, M.szulgai and M. marinum (13), but these are uncommon. Hence, manufacturers of these tests suggest a single cut-off point to define a positive test that indicates “M. tuberculosis infection likely”.
Referral for follow-up
If the TB infection test is negative the patient can usually be discharged (if asymptomatic), with counselling about potential future TB exposures and risk of infection. In some settings, although not a WHO recommendation, a second TB infection test is done 8 weeks after the end of exposure to identify delayed infections and late TB infection test conversions, particularly in the evaluation of people with significant exposure, such as household contacts of a contagious TB case.
If the TB infection test is positive the patient should be promptly referred for medical evaluation and consideration for TPT initiation. Ideally, medical evaluation, including CXR when available, should be performed on the same day as the reading of the test. This “one stop shop” approach will minimize delays in initiation of TPT, reduce losses in the TB infection cascade of care, and reduce patient costs and time lost from travel and waiting during multiple visits to a clinical facility.
Future testing
If the individual has a negative test, then they may be a candidate for future testing (if required as per national policy). If the individual has a positive test, they should not have a skin test in the future. Although skin tests can revert from positive to negative, the clinical interpretation of individuals who had a prior positive test and now have a negative or a more strongly positive test is unclear (38).
 Reacción
Reacción