Enlaces transversales de Book para A2.1 QFT-Plus – step by step
Step 1. TB screening
The first step is to screen for TB, as outlined in Step 1 for tuberculin skin test (TST) or Mycobacterium tuberculosis antigen-based skin test (TBST) in Annex 1.
Step 2. Explain the administration of QFT-Plus
It is important to ensure that the patient understands the procedures and agrees to them. The patient should be informed of the consequence of being tested, and should understand the result and what they will need to do after receiving the results.
Step 3. Collect blood and handle appropriately (up to incubation)
There are various options for blood collection for the QFT-Plus (1).
Option A: direct draw into QFT-Plus blood collection tubes
One option is to draw the blood directly into QFT-Plus blood collection tubes:
- Tubes should be labelled appropriately, with each tube identifiable by its label once the cap is removed, and the time and date of blood collection included on the label. It is also important to make sure that the tubes are kept at room temperature (17–25 °C) at the time of blood collection.
- A trained phlebotomist should collect 1 mL of blood by venepuncture directly into each of the blood collection tubes for each patient, ensuring that the correct volume is drawn – the validated range is 0.8–1.2 mL (Fig. A2.1.1). Underfilling or overfilling the tube with an amount outside the validated range may lead to erroneous results.
Fig. A2.1.1. Direct draw into QFT-Plus blood collection tubes
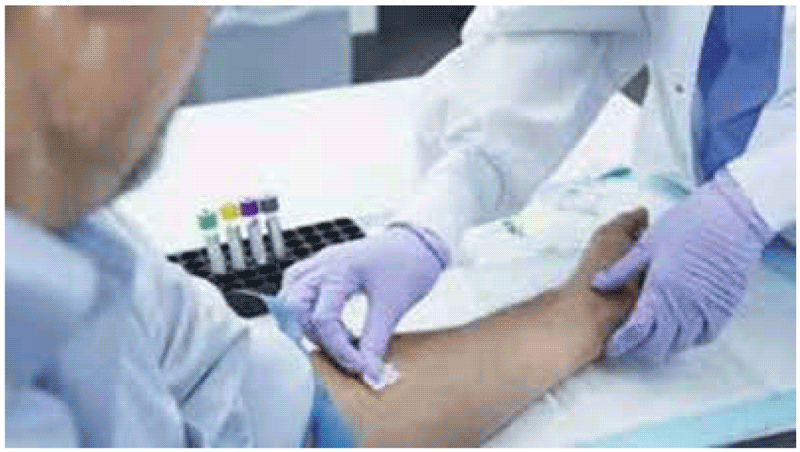
See video at:
https://www.quantiferon.com/us/products/quantiferon-tb-gold-plus-us/laboratory-resources/training-videos-labs/
- Once the tubes have been filled, they should immediately be shaken 10 times, just firmly enough to ensure that the entire inner surface of the tube is coated with blood, to dissolve antigens on the tube walls. Shaking too vigorously should be avoided as it may disrupt the gel and lead to aberrant results.
- Following shaking, the tubes must be transferred to an incubator set at 37 °C (±1 °C) as soon as possible (and within 16 hours of collection). If tubes are not incubated directly after blood collection and shaking, they should be inverted 10 times, to mix them, before being incubated at 37 °C.
- The QFT-Plus blood collection tubes should be incubated upright at 37 °C (±1 °C) for 16–24 hours. The incubator does not need CO2 or humidification. This process is shown in Fig. A2.1.2.
Fig. A2.1.2. Process for direct draw into QFT-Plus blood collection tubes
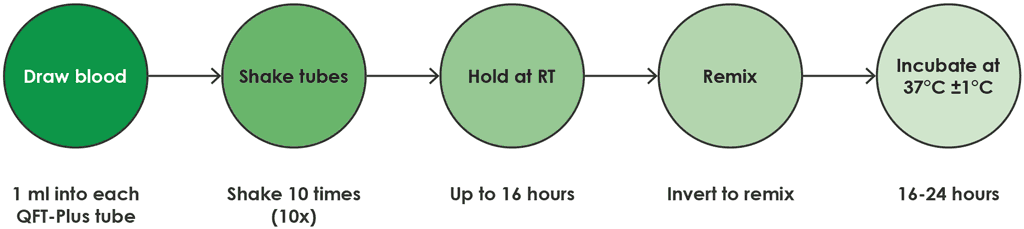
RT: room temperature.
Source: product insert (1).
Option B: blood collection into a single lithium heparin tube followed by transfer to QFT-Plus blood collection tubes
Another option is for blood to be collected in a single blood collection tube containing lithium heparin as the anticoagulant and then transferred to a QFT-Plus blood collection tube:
- Only lithium heparin should be used as a blood anticoagulant, because other anticoagulants may interfere with the assay.
- Tubes should be labelled appropriately, as for the direct draw option above.
- A trained phlebotomist should fill a lithium heparin blood collection tube (minimum volume 5 mL) (Fig. A2.1.3), then invert the tube several times to dissolve the heparin.
Fig. A2.1.3. Blood collection into a single lithium heparin tube
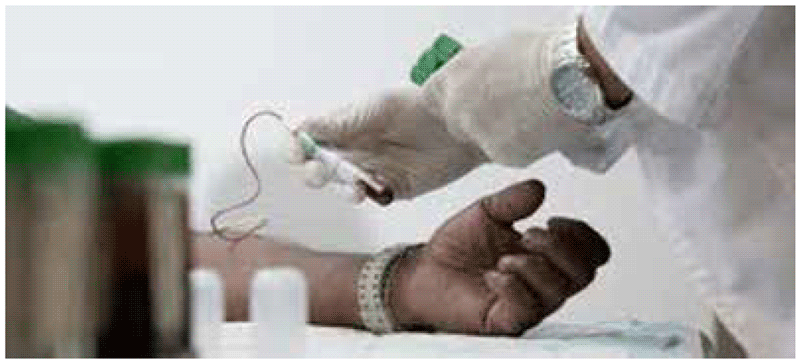
See video at:
https://www.quantiferon.com/us/products/quantiferon-tb-gold-plus-us/ laboratory-resources/training-videos-labs/
There are different options for hold time and temperature for lithium heparin tubes before transfer to QFT-Plus blood collection tubes for incubation.
Option A: room temperature storage and handling of lithium heparin tube
- In this option, the blood collected in the lithium heparin tube is maintained at room temperature (17–25 °C) for no more than 12 hours from the time of collection, before being transferred to a QFT-Plus blood collection tube and then incubated (Fig. A2.1.4).
Fig. A2.1.4. Draw into lithium heparin tube and hold at room temperature
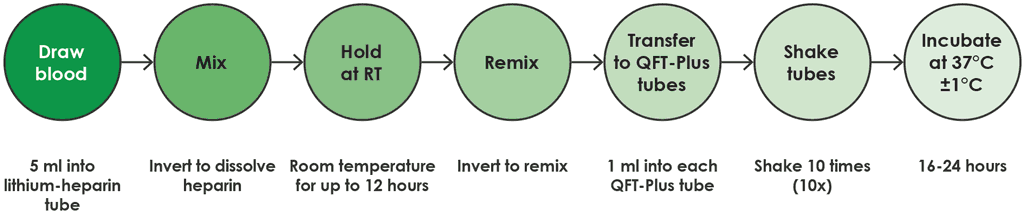
RT: room temperature.
Source: product insert (1).
Option B: refrigerated storage and handling of lithium heparin tube
- In this option, blood drawn into a lithium heparin tube may be held at room temperature (17–25 °C) for up to 3 hours after blood collection, then refrigerated (2–8 °C) for up to 48 hours.
- After refrigeration, the lithium heparin tube must equilibrate to room temperature (17–25 °C) before the sample is transferred to QFT-Plus blood collection tubes.
- Following transfer of the sample, the QFT-Plus blood collection tubes should be placed in the 37 °C incubator within 2 hours.
If there is a delay between transfer to QFT-Plus blood collection tubes and incubation at 37 °C, the tubes should be mixed 10 times (by inversion) before being incubated at 37 °C. Total time from blood draw to incubation in QFT-Plus blood collection tubes should not exceed 53 hours (Fig. A2.1.5).
Fig. A2.1.5. Draw into lithium heparin tube and hold at 2–8 °C
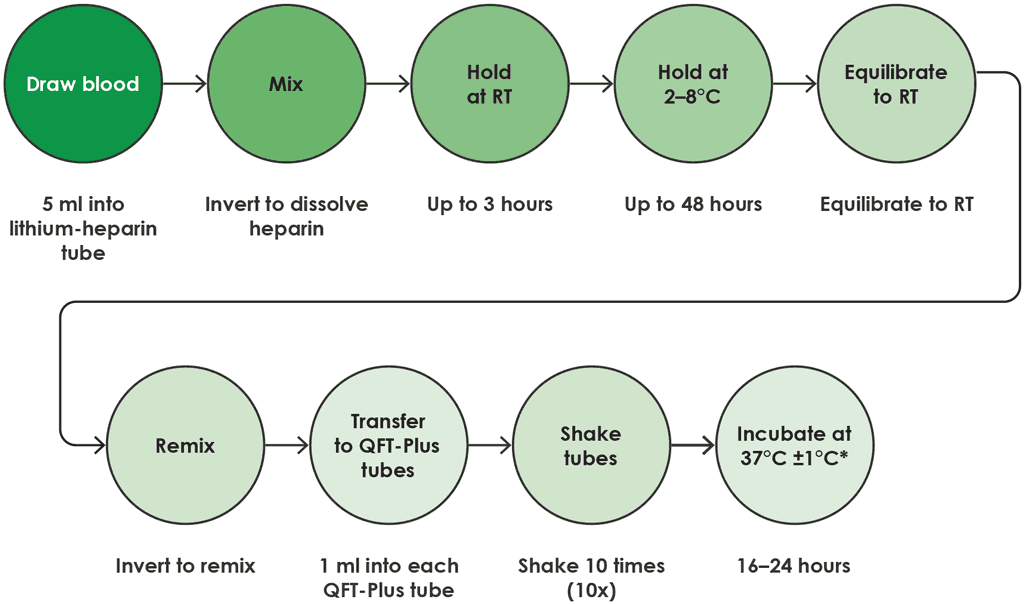
RT: room temperature.
Source: product insert (1).
- Transfer of blood specimen from a lithium heparin tube to QFT-Plus blood collection tubes:
- Each QFT-Plus blood collection tube should be labelled appropriately, ensuring that each of the four tubes (nil, mitogen, TB1 and TB2) is identifiable by its label or other means once the cap is removed.
- The sample must be evenly mixed by gentle inversion before it is dispensed into QFT-Plus blood collection tubes. Also, the dispensing should be performed aseptically, ensuring appropriate safety procedures when removing the caps from the four QFT-Plus blood collection tubes and adding 1 mL of blood to each tube.
- The tube caps should be replaced securely and the contents mixed as described below.
- Mixing of samples:
- Immediately after the QFT-Plus blood collection tubes have been filled, the tubes should be shaken 10 times, just firmly enough to make sure that the entire inner surface of the tube is coated with blood, to dissolve antigens on tube walls.
- Overly vigorous shaking should be avoided because it may disrupt the gel and lead to aberrant results.
- After mixing, the tubes must be transferred to a 37 °C ±1 °C incubator within 2 hours. If there is a delay between transfer to QFT-Plus blood collection tubes and incubation at 37 °C, the tubes should be mixed 10 times (by inversion) before being incubated at 37 °C.
- The QFT-Plus blood collection tubes should be incubated upright at 37 °C ±1 °C for 16–24 hours. The incubator does not require CO2 or humidification.
Step 4. Centrifugation
After incubation, tubes are centrifuged at 2000–3000 relative centrifugal force (RCF). The supernatant is then extracted (the manufacturer notes that this step can be done without centrifugation, but care must be taken not to aspirate any red blood cells).
Step 5. Enzyme-linked immunosorbent assay
The enzyme-linked immunosorbent assay (ELISA) involves several steps of mixing reagents with the supernatant. This mix is then incubated for 2 hours, then the plate is washed, more reagents are added and there is another 30 minutes of incubation. Finally, the concentration of interferon-gamma is estimated from the optical density, measured using an optical density plate reader.
Materials and supplies provided by manufacturer
The product insert (1) explains that two components are provided by the manufacturer: the blood collection tubes (nil, mitogen, TB1 and TB2), and the ELISA kit reagents. QFT-Plus uses two types of collection tubes:
- QFT-Plus blood collection tubes, for use between sea level and 810 m: nil tubes (grey cap with white ring), mitogen tubes (purple cap with white ring), TB1 tubes (green cap with white ring) and TB2 tubes (yellow cap with white ring); and
- high altitude (HA) QFT-Plus blood collection tubes, for use between 1020 m and 1875 m: HA nil tubes (grey cap with yellow ring), HA mitogen tubes (purple cap with yellow ring), HA TB1 tubes (green cap with yellow ring) and HA TB2 tubes (yellow cap with yellow ring).
The QFT-Plus blood collection tubes should be stored at 4–25 °C. The ELISA kit reagents should be refrigerated but not frozen, and enzyme substrate solution should always be protected from direct sunlight. Other materials required but not provided are listed below. All instruments used in the procedure must be calibrated according to the manufacturer’s recommendations.
Equipment needed in the laboratory
The following equipment is required for the QFT-Plus:
- 37 °C ±1 °C incubator (with or without CO2);
- centrifuge capable of centrifuging the blood tubes at least to 3000 RCF (g);
- microplate shaker capable of speeds of 500–1000 rpm;
- microplate washer – for safety in handling plasma samples, an automated washer is recommended;
- microplate reader fitted with 450 nm filter and 620–650 nm reference filter;
- variable speed vortex;
- timer;
- graduated cylinder (1 L or 2 L); and
- reagent reservoirs.
Supplies needed in the laboratory
The following supplies are required for the QFT-Plus:
- calibrated variable-volume pipettes for delivery of 10 μL to 1000 μL, with disposable tips;
- calibrated multichannel pipette capable of delivering 50 μL to 100 μL, with disposable tips;
- deionized or distilled water, 2 L; and
- optional: 1 mL microtubes with caps in 96-well format racks or uncoated microplates with plastic seals for plasma storage (22 patient samples per rack or plate).
Personnel needed
For blood draws, a phlebotomist is required initially; this can be a nurse, laboratory technician, physician or other allied health professional.
If the blood is drawn directly into the QFT-Plus tube, initial handling is minimal because the tubes are placed directly in the incubator.
After incubation, personnel involved in performing QFT-Plus should be qualified laboratory technicians who are trained in ELISA techniques. Substantial staff time is required for the centrifugation, pipetting and mixing of reagents and samples; second incubation; further handling, washing and mixing; third incubation; and, finally, the actual ELISA measuring (which also involves setting up a standard curve).
Training, monitoring and quality control
Staff require initial training and ongoing supervision, monitoring and evaluation for quality control (see Chapter 4). The accuracy of test results depends on the creation of an accurate ELISA standards curve; therefore, the results from the standards must be examined before test sample results can be interpreted.
Precautions
Laboratory technicians should always wear personal protective equipment (PPE). QFT-Plus is for in vitro diagnostic use only. Failure to adhere to the manufacturer’s instructions may lead to an incorrect interpretation of the results.
Interpretation of QFT-Plus
The manufacturer recommends that QFT-Plus results are interpreted based on the relative concentrations of interferon-gamma in the positive and negative controls and the TB antigen tubes (1). The concentrations are calculated based on the optic density measured at the same time from wells containing known concentrations of interferon-gamma from dilutions of a standard solution. Although results are quantitative, the manufacturer recommends that qualitative results are reported to the provider (i.e. positive, negative or indeterminate) and that approval is sought from regulatory agencies for qualitative reporting (Table A2.1.1 and Fig. A2.1.6).
Table A2.1.1. Interpretation of QFT-Plus test results
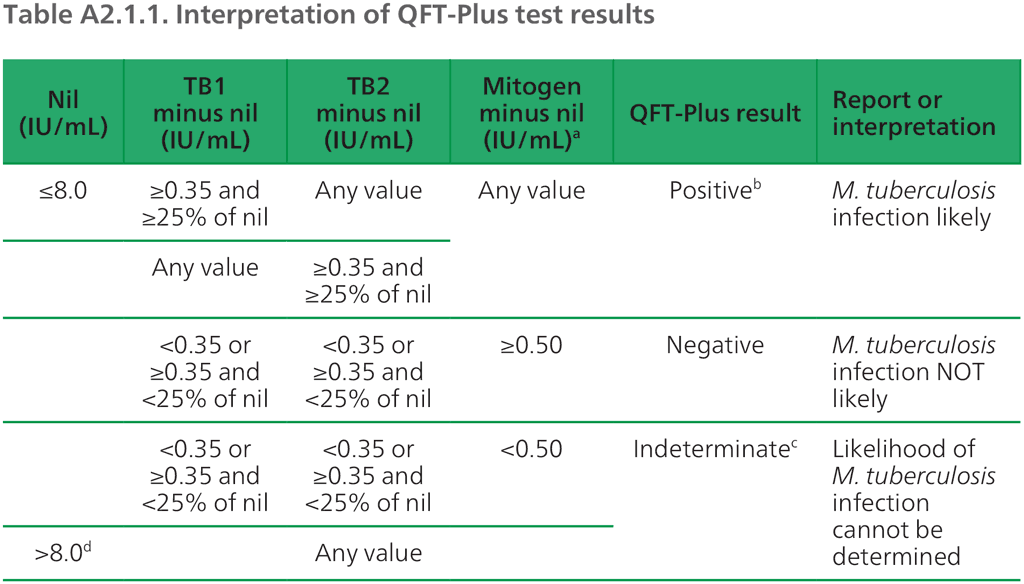
ELISA: enzyme-linked immunosorbent assay; IU: international units; M. tuberculosis: Mycobacterium tuberculosis; TB: tuberculosis
ᵃ Responses to the mitogen positive control (and occasionally to the TB antigen) can be outside the range of the microplate reader; this does not affect the test results. Values >10 IU/mL are reported by the QFT-Plus software as >10 IU/mL.
ᵇ Where M. tuberculosis infection is not suspected, initially positive results can be confirmed by re-testing the original plasma samples in duplicate in the QFT-Plus ELISA. If repeat testing of one or both replicates is positive, the test result is considered positive.
ᶜ Refer to “Troubleshooting guide” in the package insert for possible causes.
ᵈ In clinical studies, less than 0.25% of subjects had interferon-gamma levels >8.0 IU/mL for the nil value.
Source: product insert (1).
Fig. A2.1.6. Interpretation of QFT-Plus
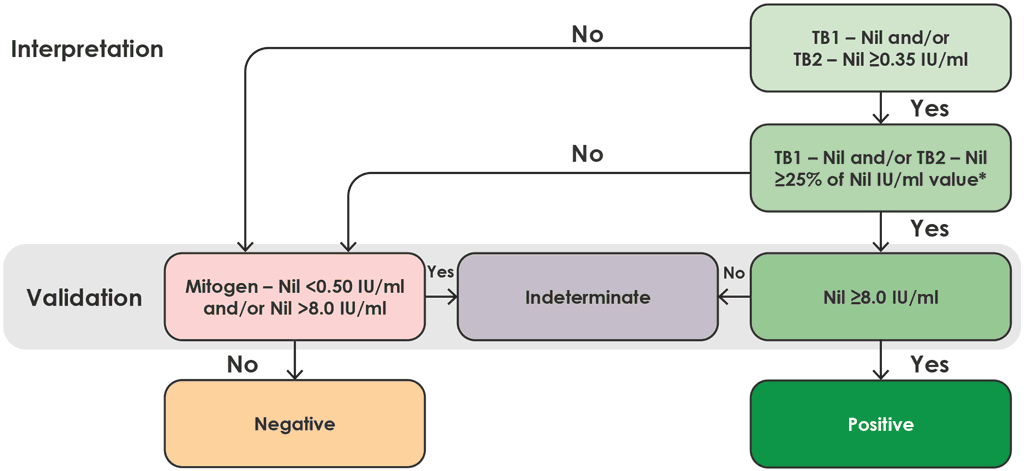
IU: international units.
Note: for TB1 minus nil or TB2 minus nil value to be valid, the ≥25% of the nil IU/mL value must be from the same tube as the original ≥0.35 IU/mL result.
Source: product insert (1).
 Reacción
Reacción